Cytoplasm and Beyond: Dynamic Innate Immune Sensing of Influenza A Virus by RIG-I
- PMID: 30760567
- PMCID: PMC6450113
- DOI: 10.1128/JVI.02299-18
Cytoplasm and Beyond: Dynamic Innate Immune Sensing of Influenza A Virus by RIG-I
Abstract
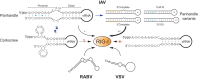
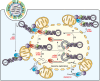
Innate immune sensing of influenza A virus (IAV) requires retinoic acid-inducible gene I (RIG-I), a fundamental cytoplasmic RNA sensor. How RIG-I's cytoplasmic localization reconciles with the nuclear replication nature of IAV is poorly understood. Recent findings provide advanced insights into the spatiotemporal RIG-I sensing of IAV and highlight the contribution of various RNA ligands to RIG-I activation. Understanding a compartment-specific RIG-I-sensing paradigm would facilitate the identification of the full spectrum of physiological RIG-I ligands produced during IAV infection.
Keywords: RIG-I; defective interfering RNA; influenza A virus; innate immunity; panhandle.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Inhibition of Ongoing Influenza A Virus Replication Reveals Different Mechanisms of RIG-I Activation.J Virol. 2019 Mar 5;93(6):e02066-18. doi: 10.1128/JVI.02066-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602605 Free PMC article.
-
Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction.J Virol. 2015 Jun;89(11):6067-79. doi: 10.1128/JVI.00232-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810557 Free PMC article.
-
Defective RNA sensing by RIG-I in severe influenza virus infection.Clin Exp Immunol. 2018 Jun;192(3):366-376. doi: 10.1111/cei.13120. Epub 2018 Mar 24. Clin Exp Immunol. 2018. PMID: 29453856 Free PMC article.
-
Innate Immune Sensing of Influenza A Virus.Viruses. 2020 Jul 14;12(7):755. doi: 10.3390/v12070755. Viruses. 2020. PMID: 32674269 Free PMC article. Review.
-
Recent developments in understanding RIG-I's activation and oligomerization.Sci Prog. 2024 Jul-Sep;107(3):368504241265182. doi: 10.1177/00368504241265182. Sci Prog. 2024. PMID: 39091074 Free PMC article. Review.
Cited by
-
Genes, inflammatory response, tolerance, and resistance to virus infections in migratory birds, bats, and rodents.Front Immunol. 2023 Aug 29;14:1239572. doi: 10.3389/fimmu.2023.1239572. eCollection 2023. Front Immunol. 2023. PMID: 37711609 Free PMC article. Review.
-
Tripartite motif-containing protein 46 accelerates influenza A H7N9 virus infection by promoting K48-linked ubiquitination of TBK1.Virol J. 2022 Nov 3;19(1):176. doi: 10.1186/s12985-022-01907-x. Virol J. 2022. PMID: 36329446 Free PMC article.
-
Secondary Structure of Influenza A Virus Genomic Segment 8 RNA Folded in a Cellular Environment.Int J Mol Sci. 2022 Feb 23;23(5):2452. doi: 10.3390/ijms23052452. Int J Mol Sci. 2022. PMID: 35269600 Free PMC article.
-
Effects of Cigarette Smoking on Influenza Virus/Host Interplay.Pathogens. 2021 Dec 17;10(12):1636. doi: 10.3390/pathogens10121636. Pathogens. 2021. PMID: 34959590 Free PMC article. Review.
-
Exosomes originating from infection with the cytoplasmic single-stranded RNA virus Rift Valley fever virus (RVFV) protect recipient cells by inducing RIG-I mediated IFN-B response that leads to activation of autophagy.Cell Biosci. 2021 Dec 25;11(1):220. doi: 10.1186/s13578-021-00732-z. Cell Biosci. 2021. PMID: 34953502 Free PMC article.
References
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi:10.1038/nature04734. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

