Maintaining extraembryonic expression allows generation of mice with severe tissue factor pathway inhibitor deficiency
- PMID: 30755437
- PMCID: PMC6373739
- DOI: 10.1182/bloodadvances.2018018853
Maintaining extraembryonic expression allows generation of mice with severe tissue factor pathway inhibitor deficiency
Abstract
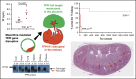
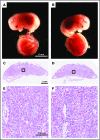
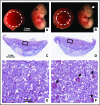
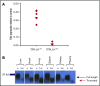
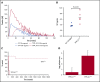
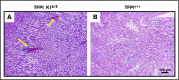
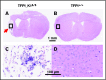
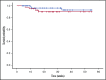
Tissue factor pathway inhibitor (TFPI) is a serine protease with multiple anticoagulant activities. The Kunitz1 (K1) domain of TFPI binds the active site of factor VIIa and is required for inhibition of tissue factor (TF)/factor VIIa catalytic activity. Mice lacking TFPI K1 domain die in utero. TFPI is highly expressed on trophoblast cells of the placenta. We used genetic strategies to selectively ablate exon 4 encoding TFPI K1 domain in the embryo, while maintaining expression in trophoblast cells. This approach resulted in expected Mendelian frequency of TFPI K1 domain-deficient mice. Real-time polymerase chain reaction confirmed 95% to 99% genetic deletion and a similar reduction in transcript expression. Western blotting confirmed the presence of a truncated protein instead of full-length TFPI. Mice with severe TFPI K1 deficiency exhibited elevated thrombin-antithrombin (TAT) levels, frequent fibrin deposition in renal medulla, and increased susceptibility to TF-induced pulmonary embolism. They were fertile, and most lived normal life spans without any overt thrombotic events. Of 43 mice observed, 2 displayed extensive brain ischemia and infarction. We conclude that in contrast to complete absence of TFPI K1 domain, severe deficiency is compatible with in utero development, adult survival, and reproductive functions in mice. Inhibition of TFPI activity is being evaluated as a means of boosting thrombin generation in hemophilia patients. Our results show that in mice severe reduction of TFPI K1 activity is associated with a prothrombotic state without overt developmental outcomes. We note fibrin deposits in the kidney and rare cases of brain ischemia.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.Z. is a current employee of MPP Group LLC. M.W.L. receives research support from and is a member of Scientific Advisory Boards for Audentes Therapeutics, Solid Biosciences, and Ichorion Therapeutics and is a consultant for Wave Life Sciences. A.E.M. receives research grant from Novo Nordisk. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
A balance between TFPI and thrombin-mediated platelet activation is required for murine embryonic development.Blood. 2015 Jun 25;125(26):4078-84. doi: 10.1182/blood-2015-03-633958. Epub 2015 May 7. Blood. 2015. PMID: 25954015 Free PMC article.
-
Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice.Blood. 1997 Aug 1;90(3):944-51. Blood. 1997. PMID: 9242522
-
Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II.J Biol Chem. 1997 Feb 14;272(7):4367-77. doi: 10.1074/jbc.272.7.4367. J Biol Chem. 1997. PMID: 9020158
-
Tissue factor pathway inhibitor gene disruption.Blood Coagul Fibrinolysis. 1998 Mar;9 Suppl 1:S89-92. Blood Coagul Fibrinolysis. 1998. PMID: 9819035 Review.
-
Structure and biology of tissue factor pathway inhibitor.Thromb Haemost. 2001 Oct;86(4):959-72. Thromb Haemost. 2001. PMID: 11686353 Review.
Cited by
-
Intrauterine lethality in Tfpi gene disrupted mice is differentially suppressed during mid- and late-gestation by platelet TFPIα overexpression.J Thromb Haemost. 2021 Jun;19(6):1483-1492. doi: 10.1111/jth.15299. Epub 2021 Apr 12. J Thromb Haemost. 2021. PMID: 33728763 Free PMC article.
-
Intersection of regulatory pathways controlling hemostasis and hemochorial placentation.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2111267118. doi: 10.1073/pnas.2111267118. Proc Natl Acad Sci U S A. 2021. PMID: 34876522 Free PMC article.
-
The Impact of Dabigatran and Rivaroxaban on Variation of Platelet Activation Biomarkers and DRT Following Percutaneous Left Atrial Appendage Closure.Front Pharmacol. 2021 Sep 15;12:723905. doi: 10.3389/fphar.2021.723905. eCollection 2021. Front Pharmacol. 2021. PMID: 34603033 Free PMC article.
-
Tissue Factor Pathway Inhibitors as Potential Targets for Understanding the Pathophysiology of Preeclampsia.Biomedicines. 2023 Apr 22;11(5):1237. doi: 10.3390/biomedicines11051237. Biomedicines. 2023. PMID: 37238908 Free PMC article. Review.
References
-
- Edstrom CS, Calhoun DA, Christensen RD. Expression of tissue factor pathway inhibitor in human fetal and placental tissues. Early Hum Dev. 2000;59(2):77-84. - PubMed
-
- Mast AE, Acharya N, Malecha MJ, Hall CL, Dietzen DJ. Characterization of the association of tissue factor pathway inhibitor with human placenta. Arterioscler Thromb Vasc Biol. 2002;22(12):2099-2104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

