Intraflagellar transport 20 promotes collective cancer cell invasion by regulating polarized organization of Golgi-associated microtubules
- PMID: 30742741
- PMCID: PMC6447847
- DOI: 10.1111/cas.13970
Intraflagellar transport 20 promotes collective cancer cell invasion by regulating polarized organization of Golgi-associated microtubules
Abstract
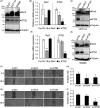
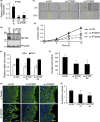
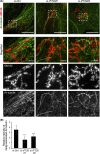
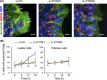
Collective invasion is an important strategy of cancers of epithelial origin, including colorectal cancer (CRC), to infiltrate efficiently into local tissues as collective cell groups. Within the groups, cells at the invasive front, called leader cells, are highly polarized and motile, thereby providing the migratory traction that guides the follower cells. However, its underlying mechanisms remain unclear. We have previously shown that signaling emanating from the receptor tyrosine kinase Ror2 can promote invasion of human osteosarcoma cells and that intraflagellar transport 20 (IFT20) mediates its signaling to regulate Golgi structure and transport. Herein, we investigated the role of Ror2 and IFT20 in collective invasion of CRC cells, where Ror2 expression is either silenced or nonsilenced. We show by cell biological analyses that IFT20 promotes collective invasion of CRC cells, irrespective of expression and function of Ror2. Intraflagellar transport 20 is required for organization of Golgi-associated, stabilized microtubules, oriented toward the direction of invasion in leader cells. Our results also indicate that IFT20 promotes reorientation of the Golgi apparatus toward the front side of leader cells. Live cell imaging of the microtubule plus-end binding protein EB1 revealed that IFT20 is required for continuous polarized microtubule growth in leader cells. These results indicate that IFT20 plays an important role in collective invasion of CRC cells by regulating organization of Golgi-associated, stabilized microtubules and Golgi polarity in leader cells.
Keywords: IFT20; Ror2; collective invasion; colorectal cancer; noncentrosomal microtubule.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness.Sci Rep. 2017 Jan 26;7(1):1. doi: 10.1038/s41598-016-0028-x. Sci Rep. 2017. PMID: 28127051 Free PMC article.
-
The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly.Mol Biol Cell. 2006 Sep;17(9):3781-92. doi: 10.1091/mbc.e06-02-0133. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775004 Free PMC article.
-
Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network.Dev Cell. 2007 Jun;12(6):917-30. doi: 10.1016/j.devcel.2007.04.002. Dev Cell. 2007. PMID: 17543864 Free PMC article.
-
Cadherin mechanotransduction in leader-follower cell specification during collective migration.Exp Cell Res. 2019 Mar 1;376(1):86-91. doi: 10.1016/j.yexcr.2019.01.006. Epub 2019 Jan 8. Exp Cell Res. 2019. PMID: 30633881 Review.
-
Microtubule organization and function in epithelial cells.Traffic. 2004 Jan;5(1):1-9. doi: 10.1111/j.1600-0854.2003.00149.x. Traffic. 2004. PMID: 14675420 Review.
Cited by
-
Actin-driven Golgi apparatus dispersal during collective migration of epithelial cells.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2204808119. doi: 10.1073/pnas.2204808119. Epub 2022 Jun 24. Proc Natl Acad Sci U S A. 2022. PMID: 35749357 Free PMC article.
-
IFT20 Mediates the Transport of Cell Migration Regulators From the Trans-Golgi Network to the Plasma Membrane in Breast Cancer Cells.Front Cell Dev Biol. 2021 Feb 26;9:632198. doi: 10.3389/fcell.2021.632198. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748116 Free PMC article.
-
The Green Valley of Drosophila melanogaster Constitutive Heterochromatin: Protein-Coding Genes Involved in Cell Division Control.Cells. 2022 Sep 29;11(19):3058. doi: 10.3390/cells11193058. Cells. 2022. PMID: 36231024 Free PMC article. Review.
-
Associations of IFT20 and GM130 protein expressions with clinicopathological features and survival of patients with lung adenocarcinoma.BMC Cancer. 2022 Jul 22;22(1):809. doi: 10.1186/s12885-022-09905-6. BMC Cancer. 2022. PMID: 35869490 Free PMC article.
-
An IFT20 mechanotrafficking axis is required for integrin recycling, focal adhesion dynamics, and polarized cell migration.Mol Biol Cell. 2020 Aug 1;31(17):1917-1930. doi: 10.1091/mbc.E20-04-0232. Epub 2020 Jun 10. Mol Biol Cell. 2020. PMID: 32520638 Free PMC article.
References
-
- Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue‐tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346‐354. - PubMed
-
- Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non‐canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645‐654. - PubMed
-
- Enomoto M, Hayakawa S, Itsukushima S, et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene. 2009;28:3197‐3208. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

