Cell cycle arrest in mitosis promotes interferon-induced necroptosis
- PMID: 30742091
- PMCID: PMC6748087
- DOI: 10.1038/s41418-019-0298-5
Cell cycle arrest in mitosis promotes interferon-induced necroptosis
Abstract
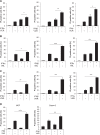
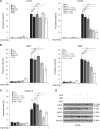
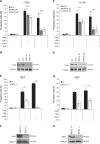
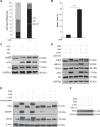
Resistance to apoptosis is a hallmark of cancer and deregulation of apoptosis often leads to chemoresistance. Therefore, new approaches to target apoptosis-resistant cancer cells are crucial for the development of directed cancer therapies. In the present study, we investigated the effect of cell cycle regulators on interferon (IFN)-induced necroptosis as an alternative cell death mechanism to overcome apoptosis resistance. Here, we report a novel combination treatment of IFNs with cell cycle arrest-inducing compounds that induce necroptosis in apoptosis-resistant cancer cells and elucidate the underlying molecular mechanisms. Combination treatment of IFNs (i.e. IFNβ) with inhibitors of the cell cycle (e.g. vinorelbine (VNR), nocodazole (Noc), polo-like kinase-1 (Plk-1) inhibitor BI 6727) co-operate to induce necroptotic cell death upon caspase inactivation. The mode of cell death was confirmed by pharmacological inhibition and siRNA-mediated downregulation of the key necroptotic factors receptor-interacting protein (RIP) kinase 3 (RIP3) and mixed-lineage kinase-like (MLKL) in various cell lines. Mechanistically, we show that necroptosis upon VNR/IFNβ/zVAD.fmk treatment is RIP1-independent but relies on IFNβ-induced gene expression of Z-DNA-binding protein 1 (ZBP1) as shown by quantitative RT-PCR and genetic knockdown experiments. Interestingly, we find that RIP3 is phosphorylated in response to compounds that trigger mitotic arrest, even in the absence of IFNβ signaling and necroptosis induction. Together, the identification of a novel combination treatment that triggers necroptosis has implications for the development of molecular-targeted therapies to circumvent apoptosis resistance and point to an underestimated role of cell cycle regulation in cell death signaling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines.Cancer Lett. 2017 Dec 1;410:228-237. doi: 10.1016/j.canlet.2017.09.002. Epub 2017 Sep 18. Cancer Lett. 2017. PMID: 28923396
-
Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked.Cancer Lett. 2016 Sep 28;380(1):31-8. doi: 10.1016/j.canlet.2016.05.036. Epub 2016 Jun 3. Cancer Lett. 2016. PMID: 27267809
-
RIP1, RIP3, and MLKL Contribute to Cell Death Caused by Clostridium perfringens Enterotoxin.mBio. 2019 Dec 17;10(6):e02985-19. doi: 10.1128/mBio.02985-19. mBio. 2019. PMID: 31848291 Free PMC article.
-
Necroptosis: Pathway diversity and characteristics.Semin Cell Dev Biol. 2015 Mar;39:56-62. doi: 10.1016/j.semcdb.2015.02.002. Epub 2015 Feb 13. Semin Cell Dev Biol. 2015. PMID: 25683283 Review.
-
RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review).Int J Mol Med. 2019 Sep;44(3):771-786. doi: 10.3892/ijmm.2019.4244. Epub 2019 Jun 14. Int J Mol Med. 2019. PMID: 31198981 Free PMC article. Review.
Cited by
-
PLK1-mediated S369 phosphorylation of RIPK3 during G2 and M phases enables its ripoptosome incorporation and activity.iScience. 2021 Mar 17;24(4):102320. doi: 10.1016/j.isci.2021.102320. eCollection 2021 Apr 23. iScience. 2021. PMID: 33870135 Free PMC article.
-
Integrated Analysis of Ferroptosis-Related Biomarker Signatures to Improve the Diagnosis and Prognosis Prediction of Ovarian Cancer.Front Cell Dev Biol. 2022 Jan 5;9:807862. doi: 10.3389/fcell.2021.807862. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071242 Free PMC article.
-
Sanguinarine Induces Necroptosis of HCC by Targeting PKM2 Mediated Energy Metabolism.Cancers (Basel). 2024 Jul 13;16(14):2533. doi: 10.3390/cancers16142533. Cancers (Basel). 2024. PMID: 39061173 Free PMC article.
-
The p53 family member p73 in the regulation of cell stress response.Biol Direct. 2021 Nov 8;16(1):23. doi: 10.1186/s13062-021-00307-5. Biol Direct. 2021. PMID: 34749806 Free PMC article. Review.
-
The Z-nucleic acid sensor ZBP1 in health and disease.J Exp Med. 2023 Aug 7;220(8):e20221156. doi: 10.1084/jem.20221156. Epub 2023 Jul 14. J Exp Med. 2023. PMID: 37450010 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

