Progression on Citrullination of Proteins in Gastrointestinal Cancers
- PMID: 30740359
- PMCID: PMC6357933
- DOI: 10.3389/fonc.2019.00015
Progression on Citrullination of Proteins in Gastrointestinal Cancers
Abstract
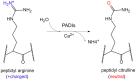
The citrullination modification (Cit) of proteins has received increasing attention in recent years. This kind of protein modification was first discovered in autoimmune diseases such as rheumatoid arthritis. The citrullination modification process is catalyzed by the peptidyl arginine deiminases (PADIs) family. A well-known citrullination of histone involves the key mechanism of neutrophil extracellular traps (NETs) of inflammation in the peripheral blood. Further studies revealed that citrullination modification of proteins also involves in carcinogenesis in human being. Citrullinated proteins disturbed the stability of proteins and caused DNA damages. There is increasing evidence that citrullinated proteins can be used as potential targets for cancer diagnosis or treatment. This review introduces the concept of citrullination modification of proteins, substrate proteins, examining methods and biological significances.
Keywords: PADIs; citrullination; histone; molecular targets; proteins.
Figures

Similar articles
-
Protein Arginine Deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination.Acc Chem Res. 2019 Mar 19;52(3):818-832. doi: 10.1021/acs.accounts.9b00024. Epub 2019 Mar 7. Acc Chem Res. 2019. PMID: 30844238 Free PMC article.
-
Histone citrullination: a new target for tumors.Mol Cancer. 2021 Jun 11;20(1):90. doi: 10.1186/s12943-021-01373-z. Mol Cancer. 2021. PMID: 34116679 Free PMC article. Review.
-
The Citrullination-Neutrophil Extracellular Trap Axis in Chronic Diseases.J Innate Immun. 2022;14(5):393-417. doi: 10.1159/000522331. Epub 2022 Mar 9. J Innate Immun. 2022. PMID: 35263752 Free PMC article. Review.
-
Citrullination: A modification important in the pathogenesis of autoimmune diseases.Clin Immunol. 2022 Dec;245:109134. doi: 10.1016/j.clim.2022.109134. Epub 2022 Sep 30. Clin Immunol. 2022. PMID: 36184053 Review.
-
Peptidyl arginine deiminases: detection and functional analysis of protein citrullination.Curr Opin Struct Biol. 2019 Dec;59:205-215. doi: 10.1016/j.sbi.2019.01.024. Epub 2019 Mar 1. Curr Opin Struct Biol. 2019. PMID: 30833201 Free PMC article. Review.
Cited by
-
Construction of a novel gene-based model for prognosis prediction of clear cell renal cell carcinoma.Cancer Cell Int. 2020 Jan 28;20:27. doi: 10.1186/s12935-020-1113-6. eCollection 2020. Cancer Cell Int. 2020. PMID: 32002016 Free PMC article.
-
Epigenetic Regulations of AhR in the Aspect of Immunomodulation.Int J Mol Sci. 2020 Sep 3;21(17):6404. doi: 10.3390/ijms21176404. Int J Mol Sci. 2020. PMID: 32899152 Free PMC article. Review.
-
PAD2 dysregulation and aberrant protein citrullination feature prominently in reactive astrogliosis and myelin protein aggregation in sporadic ALS.Neurobiol Dis. 2024 Mar;192:106414. doi: 10.1016/j.nbd.2024.106414. Epub 2024 Jan 21. Neurobiol Dis. 2024. PMID: 38253209 Free PMC article.
-
Concepts of extracellular matrix remodelling in tumour progression and metastasis.Nat Commun. 2020 Oct 9;11(1):5120. doi: 10.1038/s41467-020-18794-x. Nat Commun. 2020. PMID: 33037194 Free PMC article. Review.
-
Combined transcriptome and proteome analysis provides insights into anthocyanin accumulation in the leaves of red-leaved poplars.Plant Mol Biol. 2021 Aug;106(6):491-503. doi: 10.1007/s11103-021-01166-4. Epub 2021 Jun 24. Plant Mol Biol. 2021. PMID: 34165673
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

