The TYK2-P1104A Autoimmune Protective Variant Limits Coordinate Signals Required to Generate Specialized T Cell Subsets
- PMID: 30740104
- PMCID: PMC6355696
- DOI: 10.3389/fimmu.2019.00044
The TYK2-P1104A Autoimmune Protective Variant Limits Coordinate Signals Required to Generate Specialized T Cell Subsets
Abstract
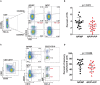
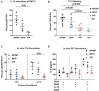
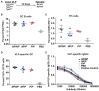
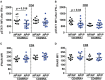
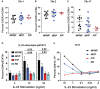
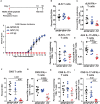
TYK2 is a JAK family member that functions downstream of multiple cytokine receptors. Genome wide association studies have linked a SNP (rs34536443) within TYK2 encoding a Proline to Alanine substitution at amino acid 1104, to protection from multiple autoimmune diseases including systemic lupus erythematosus (SLE) and multiple sclerosis (MS). The protective role of this SNP in autoimmune pathogenesis, however, remains incompletely understood. Here we found that T follicular helper (Tfh) cells, switched memory B cells, and IFNAR signaling were decreased in healthy individuals that expressed the protective variant TYK2A1104 (TYK2P ). To study this variant in vivo, we developed a knock-in murine model of this allele. Murine Tyk2P expressing T cells homozygous for the protective allele, but not cells heterozygous for this change, manifest decreased IL-12 receptor signaling, important for Tfh lineage commitment. Further, homozygous Tyk2P T cells exhibited diminished in vitro Th1 skewing. Surprisingly, despite these signaling changes, in vivo formation of Tfh and GC B cells was unaffected in two models of T cell dependent immune responses and in two alternative SLE models. TYK2 is also activated downstream of IL-23 receptor engagement. Here, we found that Tyk2P expressing T cells had reduced IL-23 dependent signaling as well as a diminished ability to skew toward Th17 in vitro. Consistent with these findings, homozygous, but not heterozygous, Tyk2P mice were fully protected in a murine model of MS. Homozygous Tyk2P mice had fewer infiltrating CD4+ T cells within the CNS. Most strikingly, homozygous mice had a decreased proportion of IL-17+/IFNγ+, double positive, pathogenic CD4+ T cells in both the draining lymph nodes (LN) and CNS. Thus, in an autoimmune model, such as EAE, impacted by both altered Th1 and Th17 signaling, the Tyk2P allele can effectively shield animals from disease. Taken together, our findings suggest that TYK2P diminishes IL-12, IL-23, and IFN I signaling and that its protective effect is most likely manifest in the setting of autoimmune triggers that concurrently dysregulate at least two of these important signaling cascades.
Keywords: IFNAR; IL-12; IL-23; TYK2; Tfh; autoimmunity; germinal center; lupus.
Figures

Similar articles
-
Tyrosine kinase 2 plays critical roles in the pathogenic CD4 T cell responses for the development of experimental autoimmune encephalomyelitis.J Immunol. 2009 Dec 1;183(11):7539-46. doi: 10.4049/jimmunol.0902740. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917699
-
IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis.J Immunol. 2015 Sep 1;195(5):1974-83. doi: 10.4049/jimmunol.1403135. Epub 2015 Jul 29. J Immunol. 2015. PMID: 26223651 Free PMC article.
-
Role of TFH Cells in Promoting T Helper 17-Induced Neuroinflammation.Front Immunol. 2018 Feb 27;9:382. doi: 10.3389/fimmu.2018.00382. eCollection 2018. Front Immunol. 2018. PMID: 29535739 Free PMC article.
-
Abnormalities of the type I interferon signaling pathway in lupus autoimmunity.Cytokine. 2021 Oct;146:155633. doi: 10.1016/j.cyto.2021.155633. Epub 2021 Jul 30. Cytokine. 2021. PMID: 34340046 Free PMC article. Review.
-
Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis?Biochim Biophys Acta. 2011 Feb;1812(2):246-51. doi: 10.1016/j.bbadis.2010.05.012. Epub 2010 Jun 18. Biochim Biophys Acta. 2011. PMID: 20600875 Free PMC article. Review.
Cited by
-
Investigating the shared genetic architecture between hypothyroidism and rheumatoid arthritis.Front Immunol. 2024 Jan 25;14:1286491. doi: 10.3389/fimmu.2023.1286491. eCollection 2023. Front Immunol. 2024. PMID: 38332917 Free PMC article.
-
TYK2 in Cancer Metastases: Genomic and Proteomic Discovery.Cancers (Basel). 2021 Aug 19;13(16):4171. doi: 10.3390/cancers13164171. Cancers (Basel). 2021. PMID: 34439323 Free PMC article. Review.
-
Association of TYK2 polymorphisms with autoimmune diseases: A comprehensive and updated systematic review with meta-analysis.Genet Mol Biol. 2021 May 3;44(2):e20200425. doi: 10.1590/1678-4685-GMB-2020-0425. eCollection 2021. Genet Mol Biol. 2021. PMID: 33949620 Free PMC article.
-
IL-23/IL-17 Axis in Inflammatory Rheumatic Diseases.Clin Rev Allergy Immunol. 2021 Feb;60(1):31-45. doi: 10.1007/s12016-020-08823-4. Epub 2020 Nov 13. Clin Rev Allergy Immunol. 2021. PMID: 33185790 Free PMC article. Review.
-
Decoding IL-23 Signaling Cascade for New Therapeutic Opportunities.Cells. 2020 Sep 7;9(9):2044. doi: 10.3390/cells9092044. Cells. 2020. PMID: 32906785 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

