Stromal cell-derived factor-1α signals via the endothelium to protect the heart against ischaemia-reperfusion injury
- PMID: 30738798
- PMCID: PMC6408335
- DOI: 10.1016/j.yjmcc.2019.02.002
Stromal cell-derived factor-1α signals via the endothelium to protect the heart against ischaemia-reperfusion injury
Abstract
Aims: The chemokine stromal derived factor-1α (SDF-1α) is known to protect the heart acutely from ischaemia-reperfusion injury via its cognate receptor, CXCR4. However, the timing and cellular location of this effect, remains controversial.
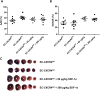
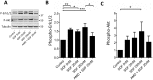
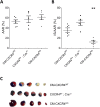
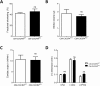
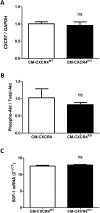
Methods and results: Wild type male and female mice were subjected to 40 min LAD territory ischaemia in vivo and injected with either saline (control) or SDF-1α prior to 2 h reperfusion. Infarct size as a proportion of area at risk was assessed histologically using Evans blue and triphenyltetrazolium chloride. Our results confirm the cardioprotective effect of exogenous SDF-1α in mouse ischaemia-reperfusion injury and, for the first time, show protection when SDF-1α is delivered just prior to reperfusion, which has important therapeutic implications. The role of cell type was examined using the same in vivo ischaemia-reperfusion protocol in cardiomyocyte- and endothelial-specific CXCR4-null mice, and by Western blot analysis of endothelial cells treated in vitro. These experiments demonstrated that the acute infarct-sparing effect is mediated by endothelial cells, possibly via the signalling kinases Erk1/2 and PI3K/Akt. Unexpectedly, cardiomyocyte-specific deletion of CXCR4 was found to be cardioprotective per se. RNAseq analysis indicated altered expression of the mitochondrial protein co-enzyme Q10b in these mice.
Conclusions: Administration of SDF-1α is cardioprotective when administered prior to reperfusion and may, therefore, have clinical utility. SDF-1α-CXCR4-mediated cardioprotection from ischaemia-reperfusion injury is contingent on the cellular location of CXCR4 activation. Specifically, cardioprotection is mediated by endothelial signalling, while cardiomyocyte-specific deletion of CXCR4 has an infarct-sparing effect per se.
Keywords: CXCR4; Cardiomyocyte; Cardioprotection; Endothelial; Ischaemia-reperfusion injury; SDF-1α.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

 NS.
NS.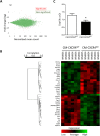

Similar articles
-
Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis.Circulation. 2007 Aug 7;116(6):654-63. doi: 10.1161/CIRCULATIONAHA.106.672451. Epub 2007 Jul 23. Circulation. 2007. PMID: 17646584 Free PMC article.
-
SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury.Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1496-505. doi: 10.1152/ajpheart.00365.2011. Epub 2011 Aug 5. Am J Physiol Heart Circ Physiol. 2011. PMID: 21821779 Free PMC article.
-
MicroRNA-668-3p Protects Against Oxygen-Glucose Deprivation in a Rat H9c2 Cardiomyocyte Model of Ischemia-Reperfusion Injury by Targeting the Stromal Cell-Derived Factor-1 (SDF-1)/CXCR4 Signaling Pathway.Med Sci Monit. 2020 Jan 30;26:e919601. doi: 10.12659/MSM.919601. Med Sci Monit. 2020. PMID: 31997826 Free PMC article.
-
Transfusion of CXCR4-primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice.PLoS One. 2012;7(11):e50105. doi: 10.1371/journal.pone.0050105. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185548 Free PMC article.
-
Stromal derived factor 1α: a chemokine that delivers a two-pronged defence of the myocardium.Pharmacol Ther. 2014 Sep;143(3):305-15. doi: 10.1016/j.pharmthera.2014.03.009. Epub 2014 Apr 1. Pharmacol Ther. 2014. PMID: 24704323 Free PMC article. Review.
Cited by
-
FAM3A - A mitochondrial route to the stimulation of angiogenesis?EBioMedicine. 2019 May;43:3-4. doi: 10.1016/j.ebiom.2019.04.033. Epub 2019 Apr 24. EBioMedicine. 2019. PMID: 31029586 Free PMC article. No abstract available.
-
The RISK pathway leading to mitochondria and cardioprotection: how everything started.Basic Res Cardiol. 2023 May 26;118(1):22. doi: 10.1007/s00395-023-00992-5. Basic Res Cardiol. 2023. PMID: 37233787 Free PMC article. Review.
-
Side Effects of Coronary Stenting such as Severe Coronary Stenosis and Multiple Coronary Chronic Total Occlusions in Elderly Patients via Induced Proinflammatory and Prooxidative Stress.Mediators Inflamm. 2019 Nov 3;2019:7147652. doi: 10.1155/2019/7147652. eCollection 2019. Mediators Inflamm. 2019. PMID: 31780868 Free PMC article.
-
Superiority of Adipose-derived CD34 + Cells over Adipose-derived Stem Cells in Promoting Ischemic Tissue Survival.Stem Cell Rev Rep. 2022 Feb;18(2):660-671. doi: 10.1007/s12015-021-10276-x. Epub 2021 Nov 17. Stem Cell Rev Rep. 2022. PMID: 34787794
-
Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential.Basic Res Cardiol. 2020 Mar 7;115(3):26. doi: 10.1007/s00395-020-0785-3. Basic Res Cardiol. 2020. PMID: 32146560 Free PMC article.
References
-
- Gibson C.M. NRMI and current treatment patterns for ST-elevation myocardial infarction. Am. Heart J. 2004;148(5 Suppl):S29–S33. - PubMed
-
- Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. - PubMed
-
- Lonborg J., Vejlstrup N., Kelbaek H., Holmvang L., Jorgensen E., Helqvist S., Saunamaki K., Ahtarovski K.A., Botker H.E., Kim W.Y., Clemmensen P., Engstrom T. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: an observational study. Eur. Heart J. Cardiovasc. Imaging. 2013;14(4):387–395. - PubMed
-
- Koudstaal S., Pujades-Rodriguez M., Denaxas S., Gho J.M., Shah A.D., Yu N., Patel R.S., Gale C.P., Hoes A.W., Cleland J.G., Asselbergs F.W., Hemingway H. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population-based linked electronic health record cohort study in 2.1 million people. Eur. J. Heart Fail. 2017;19(9):1119–1127. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

