Functional analysis of apple stem pitting virus coat protein variants
- PMID: 30736799
- PMCID: PMC6368714
- DOI: 10.1186/s12985-019-1126-8
Functional analysis of apple stem pitting virus coat protein variants
Abstract
Background: Although the canonical function of viral coat protein (CP) is to encapsidate the viral genome, they have come to be recognized as multifunctional proteins, involved in almost every stage of the viral infection cycle. However, CP functions of Apple stem pitting virus (ASPV) has not been comprehensively documented. This study aimed to characterize the functions of ASPV CP and any functional diversification caused by sequence diversity of six ASPV CP variants and studied their biological, serological, pathogenic and viral suppressor of RNA silencing (VSR) functions.
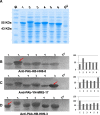
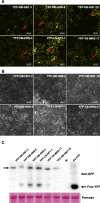
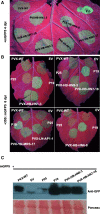
Methods: Six ASPV CP variants that have previously been shown to belong to different subgroups were selected here to study their diversity functions. Agrobacterium mediated infiltration (Agroinfiltration) was used to express YFP-ASPV-CPs in Nicotiana. benthamiana and infect Nicotiana. occidental with PVX-ASPV-CPs in. Confocal microscopy was used to detect YFP-ASPV-CPs florescence. CPs expressed in Escherichia coli BL21 (DE3) were induced by IPTG.
Results: In this study, we showed that recombinant CPs expressed in Escherichia coli BL21 (DE3) had different levels of serological reactivity to three anti-ASPV antibodies used to detect ASPV. Furthermore, fusion CPs with YFP (YFP-CPs) expressed in N. benthamiana cells differed in their ability to form aggregates. We also showed that ASPV isolates that harbour these CPs induced different biological symptoms on its herbaceous host N. occidentalis. At the same time, we found that all six CPs when expressed in PVX vector showed similar VSR activity and produced similar symptoms in N. occidentalis, despite their differences in amino acids.
Conclusions: Different ASPV isolates induced different symptoms in N. occidentalis, however, ASPV CP variants expressed in PVX vector showed the same symptoms in N. occidentalis plants. Also, we showed that ASPV CP variants has the same level of VSR activity, but they have different abilities to aggregate in N. benthamiana.
Keywords: Aggregate; Apple stem pitting virus; CP variants; Coat protein; RNA silencing suppressor.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Analysis of the complete genome of peach chlorotic mottle virus: identification of non-AUG start codons, in vitro coat protein expression, and elucidation of serological cross-reactions.Arch Virol. 2007;152(12):2207-15. doi: 10.1007/s00705-007-1050-x. Epub 2007 Sep 22. Arch Virol. 2007. PMID: 17891331
-
Evolving by deleting: patterns of molecular evolution of Apple stem pitting virus isolates from Poland.J Gen Virol. 2019 Oct;100(10):1442-1456. doi: 10.1099/jgv.0.001290. Epub 2019 Aug 19. J Gen Virol. 2019. PMID: 31424379
-
Sequence diversity and potential recombination events in the coat protein gene of Apple stem pitting virus.Virus Res. 2011 Jun;158(1-2):263-7. doi: 10.1016/j.virusres.2011.03.003. Epub 2011 Mar 23. Virus Res. 2011. PMID: 21402110
-
The functions of triple gene block proteins and coat protein of apple stem pitting virus in viral cell-to-cell movement.Mol Plant Pathol. 2024 Jan;25(1):e13392. doi: 10.1111/mpp.13392. Epub 2023 Oct 13. Mol Plant Pathol. 2024. PMID: 37837244 Free PMC article.
-
Coat proteins, host factors and plant viral replication.Curr Opin Virol. 2012 Dec;2(6):712-8. doi: 10.1016/j.coviro.2012.10.001. Epub 2012 Nov 2. Curr Opin Virol. 2012. PMID: 23122854 Review.
Cited by
-
The coat protein of citrus yellow vein clearing virus directly targets the ascorbate peroxidase 1 in lemon (ClAPX1) to facilitate virus accumulation.Front Plant Sci. 2023 Nov 28;14:1306580. doi: 10.3389/fpls.2023.1306580. eCollection 2023. Front Plant Sci. 2023. PMID: 38093999 Free PMC article.
-
Camellia ringspot-associated virus 4, a proposed new foveavirus from Camellia japonica.Arch Virol. 2020 Jul;165(7):1707-1710. doi: 10.1007/s00705-020-04655-x. Epub 2020 May 14. Arch Virol. 2020. PMID: 32409876
-
Molecular Characterization of the Coat Protein Gene of Greek Apple Stem Pitting Virus Isolates: Evolution through Deletions, Insertions, and Recombination Events.Plants (Basel). 2021 May 3;10(5):917. doi: 10.3390/plants10050917. Plants (Basel). 2021. PMID: 34063623 Free PMC article.
-
Serological and molecular analysis indicates the presence of distinct viral genotypes of Apple stem pitting virus in India.3 Biotech. 2021 Jun;11(6):278. doi: 10.1007/s13205-021-02798-5. Epub 2021 May 19. 3 Biotech. 2021. PMID: 34040927 Free PMC article.
-
Loquat (Eriobotrya japonica) is a New Natural Host of Apple Stem Pitting Virus.Plants (Basel). 2020 Nov 13;9(11):1560. doi: 10.3390/plants9111560. Plants (Basel). 2020. PMID: 33202713 Free PMC article.
References
-
- Jelkmann W. Nucleotide sequences of apple stem pitting virus and of the coat protein gene of a similar virus from pear associated with vein yellows disease and their relationship with potex- and carlaviruses. J Gen Virol. 1994;75:1535–1542. - PubMed
-
- Mathioudakis MM, Maliogka VI, Dovas CI, Vasilakakis M, Katis NI. First record of the apple stem pitting virus (ASPV) in quince in Greece. J Plant Pathol. 2006;88:225.
-
- Mathioudakis MM, Maliogka VI, Katsiani AT, Katis NI. Incidence and molecular variability of apple stem pitting and apple chlorotic leaf spot viruses in apple and pear orchards in Greece. J Plant Pathol. 2010;92:139–147.
-
- Wu Z, Ku H, Su C, Chen I, Jan F. Molecular and biological characterization of an isolate of apple stem pitting virus causing pear vein yellows disease in Taiwan. J Plant Pathol. 2010;92:721–728.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

