Predicting the direct and indirect impacts of climate change on malaria in coastal Kenya
- PMID: 30726279
- PMCID: PMC6364917
- DOI: 10.1371/journal.pone.0211258
Predicting the direct and indirect impacts of climate change on malaria in coastal Kenya
Abstract
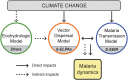
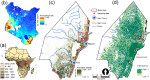
Background: The transmission of malaria is highly variable and depends on a range of climatic and anthropogenic factors. This study investigates the combined, i.e. direct and indirect, impacts of climate change on the dynamics of malaria through modifications in: (i) the sporogonic cycle of Plasmodium induced by air temperature increase, and (ii) the life cycle of Anopheles vector triggered by changes in natural breeding habitat arising from the altered moisture dynamics resulting from acclimation responses of vegetation under climate change. The study is performed for a rural region in Kilifi county, Kenya.
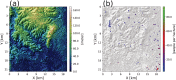
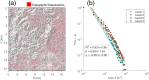
Methods and findings: We use a stochastic lattice-based malaria (SLIM) model to make predictions of changes in Anopheles vector abundance, the life cycle of Plasmodium parasites, and thus malaria transmission under projected climate change in the study region. SLIM incorporates a nonlinear temperature-dependence of malaria parasite development to estimate the extrinsic incubation period of Plasmodium. It is also linked with a spatially distributed eco-hydrologic modeling framework to capture the impacts of climate change on soil moisture dynamics, which served as a key determinant for the formation and persistence of mosquito larval habitats on the land surface. Malaria incidence data collected from 2008 to 2013 is used for SLIM model validation. Projections of climate change and human population for the region are used to run the models for prediction scenarios. Under elevated atmospheric CO2 concentration ([CO2]) only, modeled results reveal wetter soil moisture in the root zone due to the suppression of transpiration from vegetation acclimation, which increases the abundance of Anopheles vectors and the risk of malaria. When air temperature increases are also considered along with elevated [CO2], the life cycle of Anopheles vector and the extrinsic incubation period of Plasmodium parasites are shortened nonlinearly. However, the reduction of soil moisture resulting from higher evapotranspiration due to air temperature increase also reduces the larval habitats of the vector. Our findings show the complicated role of vegetation acclimation under elevated [CO2] on malaria dynamics and indicate an indirect but ignored impact of air temperature increase on malaria transmission through reduction in larval habitats and vector density.
Conclusions: Vegetation acclimation triggered by elevated [CO2] under climate change increases the risk of malaria. In addition, air temperature increase under climate change has opposing effects on mosquito larval habitats and the life cycles of both Anopheles vectors and Plasmodium parasites. The indirect impacts of temperature change on soil moisture dynamics are significant and should be weighed together with the direct effects of temperature change on the life cycles of mosquitoes and parasites for future malaria prediction and control.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Stochastic lattice-based modelling of malaria dynamics.Malar J. 2018 Jul 5;17(1):250. doi: 10.1186/s12936-018-2397-z. Malar J. 2018. PMID: 29976221 Free PMC article.
-
Malaria smear positivity among Kenyan children peaks at intermediate temperatures as predicted by ecological models.Parasit Vectors. 2019 Jun 6;12(1):288. doi: 10.1186/s13071-019-3547-z. Parasit Vectors. 2019. PMID: 31171037 Free PMC article.
-
Seasonal malaria vector and transmission dynamics in western Burkina Faso.Malar J. 2019 Apr 2;18(1):113. doi: 10.1186/s12936-019-2747-5. Malar J. 2019. PMID: 30940141 Free PMC article.
-
Climate, environment and transmission of malaria.Infez Med. 2016 Jun 1;24(2):93-104. Infez Med. 2016. PMID: 27367318 Review.
-
Rethinking the extrinsic incubation period of malaria parasites.Parasit Vectors. 2018 Mar 12;11(1):178. doi: 10.1186/s13071-018-2761-4. Parasit Vectors. 2018. PMID: 29530073 Free PMC article. Review.
Cited by
-
Epidemiology of malaria in saravan city and its suburbs from 2018 to 2023, Southeast Iran.J Res Med Sci. 2024 Aug 2;29:50. doi: 10.4103/jrms.jrms_781_23. eCollection 2024. J Res Med Sci. 2024. PMID: 39403230 Free PMC article.
-
Potential impact of climatic factors on malaria in Rwanda between 2012 and 2021: a time-series analysis.Malar J. 2024 Sep 10;23(1):274. doi: 10.1186/s12936-024-05097-5. Malar J. 2024. PMID: 39256741 Free PMC article.
-
Range dynamics of Anopheles mosquitoes in Africa suggest a significant increase in the malaria transmission risk.Ecol Evol. 2024 Jul 31;14(8):e70059. doi: 10.1002/ece3.70059. eCollection 2024 Aug. Ecol Evol. 2024. PMID: 39091337 Free PMC article.
-
Pathogen prospecting of museums: Reconstructing malaria epidemiology.Proc Natl Acad Sci U S A. 2024 Apr 9;121(15):e2310859121. doi: 10.1073/pnas.2310859121. Epub 2024 Mar 25. Proc Natl Acad Sci U S A. 2024. PMID: 38527214 Free PMC article.
-
Investigating the Impact of Irrigation on Malaria Vector Larval Habitats and Transmission Using a Hydrology-Based Model.Geohealth. 2023 Dec 10;7(12):e2023GH000868. doi: 10.1029/2023GH000868. eCollection 2023 Dec. Geohealth. 2023. PMID: 38089068 Free PMC article.
References
-
- MacDonald G. The Epidemiology and Control of Malaria. Oxford Medical Publications; United Kingdom: Oxford University Press; 1957.
-
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control Dynamics and Control. United Kingdom: Oxford University Press; 1992.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

