lncRNA PLAC2 activated by H3K27 acetylation promotes cell proliferation and invasion via the activation of Wnt/β‑catenin pathway in oral squamous cell carcinoma
- PMID: 30720068
- PMCID: PMC6411352
- DOI: 10.3892/ijo.2019.4707
lncRNA PLAC2 activated by H3K27 acetylation promotes cell proliferation and invasion via the activation of Wnt/β‑catenin pathway in oral squamous cell carcinoma
Abstract
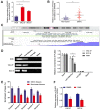
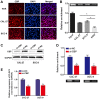
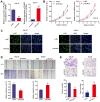
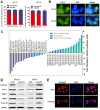
As a new group of important effector molecules involved in multiple cancer types, including breast cancer, lung cancer and oral squamous cell carcinoma, long noncoding RNAs (lncRNAs) have attracted considerable attention recently. However, the underlying cause that induces the dysregulated lncRNAs in cancer remains poorly understood. In the present study, the regulatory model of the lncRNA placenta‑specific protein 2 (PLAC2) upregulation in oral squamous cell carcinoma (OSCC) was investigated and its biological functions in OSCC malignant progression was identified. A reverse transcription‑quantitative polymerase chain reaction assay identified that PLAC2 is upregulated in OSCC cell lines and primary tissue samples. Furthermore, bioinformatic analysis followed by chromatin immunoprecipitation verified an enriched histone H3 on lysine 27 (H3K27) acetylation (H3K27ac) at the promoter region of the PLAC2 gene. Knockdown of cAMP‑response element binding protein‑binding protein (CBP) significantly reduced the enrichment level of H3K27ac, and thereby induced a decreased expression of PLAC2. Functionally, overexpression of PLAC2 promotes OSCC cell proliferation, migration and invasion, whereas knockdown of PLAC2 exerted an opposite effect. Furthermore, the Wnt/β‑catenin signaling pathway was activated by PLAC2 and mediated the PLAC2‑induced malignant progress of OSCC. In conclusion, the present results indicated that lncRNA PLAC2 is transcriptionally activated by H3K27ac modification at the promoter region in OSCC, and promotes cell growth and metastasis via activating Wnt/β‑catenin signaling pathway. Therefore, PLAC2 may serve as a promising biomarker for OSCC prognosis and therapy.
Keywords: oral squamous cell carcinoma; placenta-specific protein 2; histone H3 on lysine 27 acetylation; proliferation; invasion; Wnt/β-catenin.
Figures

Similar articles
-
Long non-coding RNA CCAT1 is a prognostic biomarker for the progression of oral squamous cell carcinoma via miR-181a-mediated Wnt/β-catenin signaling pathway.Cell Cycle. 2019 Nov;18(21):2902-2913. doi: 10.1080/15384101.2019.1662257. Epub 2019 Sep 4. Cell Cycle. 2019. PMID: 31599709 Free PMC article.
-
LncRNA AC007271.3 promotes cell proliferation, invasion, migration and inhibits cell apoptosis of OSCC via the Wnt/β-catenin signaling pathway.Life Sci. 2019 Dec 15;239:117087. doi: 10.1016/j.lfs.2019.117087. Epub 2019 Nov 20. Life Sci. 2019. PMID: 31759044
-
LINC00941 promotes oral squamous cell carcinoma progression via activating CAPRIN2 and canonical WNT/β-catenin signaling pathway.J Cell Mol Med. 2020 Sep;24(18):10512-10524. doi: 10.1111/jcmm.15667. Epub 2020 Jul 21. J Cell Mol Med. 2020. PMID: 32691935 Free PMC article.
-
Novel insights on oral squamous cell carcinoma management using long non-coding RNAs.Oncol Res. 2024 Sep 18;32(10):1589-1612. doi: 10.32604/or.2024.052120. eCollection 2024. Oncol Res. 2024. PMID: 39308526 Free PMC article. Review.
-
Unveiling the nexus: Long non-coding RNAs and the PI3K/Akt pathway in oral squamous cell carcinoma.Pathol Res Pract. 2024 Oct;262:155540. doi: 10.1016/j.prp.2024.155540. Epub 2024 Aug 12. Pathol Res Pract. 2024. PMID: 39142241 Review.
Cited by
-
Regulation of epithelial-mesenchymal transition by protein lysine acetylation.Cell Commun Signal. 2022 Apr 28;20(1):57. doi: 10.1186/s12964-022-00870-y. Cell Commun Signal. 2022. PMID: 35484625 Free PMC article. Review.
-
Plasma Circulating Terminal Differentiation-Induced Non-Coding RNA Serves as a Biomarker in Breast Cancer.Int J Hematol Oncol Stem Cell Res. 2024 Jan 1;18(1):1-6. doi: 10.18502/ijhoscr.v18i1.14739. Int J Hematol Oncol Stem Cell Res. 2024. PMID: 38680708 Free PMC article.
-
Head and neck cancer: pathogenesis and targeted therapy.MedComm (2020). 2024 Aug 21;5(9):e702. doi: 10.1002/mco2.702. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39170944 Free PMC article. Review.
-
Cudraxanthone D Regulates Epithelial-Mesenchymal Transition by Autophagy Inhibition in Oral Squamous Cell Carcinoma Cell Lines.Evid Based Complement Alternat Med. 2019 Oct 31;2019:5213028. doi: 10.1155/2019/5213028. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31781271 Free PMC article.
-
TINCR: An lncRNA with dual functions in the carcinogenesis process.Noncoding RNA Res. 2020 Jul 9;5(3):109-115. doi: 10.1016/j.ncrna.2020.06.003. eCollection 2020 Sep. Noncoding RNA Res. 2020. PMID: 32695943 Free PMC article.
References
-
- Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai D, et al. Hypoxia stimulates the cytoplasmic localization of oncogenic long noncoding RNA LINC00152 in colorectal cancer. Int J Oncol. 2018;52:453–460. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

