Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells
- PMID: 30717256
- PMCID: PMC6410246
- DOI: 10.3390/pharmaceutics11020063
Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells
Abstract
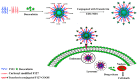
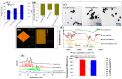
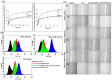
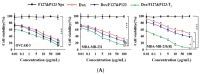
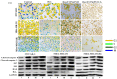
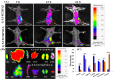
In this study, a transferrin (Tf)-conjugated polymeric nanoparticle was developed for the targeted delivery of the chemotherapeutic agent doxorubicin (Dox) in order to overcome multi-drug resistance in cancer treatment. Our objective was to improve Dox delivery for producing significant antitumor efficacy in Dox-resistant (R) breast cancer cell lines with minimum toxicity to healthy cells. The results of our experiments revealed that Dox was successfully loaded inside a transferrin (Tf)-conjugated polymeric nanoparticle composed of poloxamer 407 (F127) and 123 (P123) (Dox/F127&P123-Tf), which produced nanosized particles (~90 nm) with a low polydispersity index (~0.23). The accelerated and controlled release profiles of Dox from the nanoparticles were characterized in acidic and physiological pH and Dox/F127&P123-Tf enhanced Dox cytotoxicity in OVCAR-3, MDA-MB-231, and MDA-MB-231(R) cell lines through induction of cellular apoptosis. Moreover, Dox/F127&P123-Tf inhibited cell migration and altered the cell cycle patterns of different cancer cells. In vivo study in MDA-MB-231(R) tumor-bearing mice demonstrated enhanced delivery of nanoparticles to the tumor site when coated in a targeting moiety. Therefore, Dox/F127&P123-Tf has been tailored, using the principles of nanotherapeutics, to overcome drug-resistant chemotherapy.
Keywords: doxorubicin; doxorubicin-resistant cancer; polymeric nanoparticles; transferrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Inhibiting Multidrug Resistance with Transferrin-Targeted Polymersomes through Optimization of Ligand Density.Langmuir. 2023 Nov 14;39(45):15920-15931. doi: 10.1021/acs.langmuir.3c01726. Epub 2023 Nov 3. Langmuir. 2023. PMID: 37922445
-
Novel transferrin modified and doxorubicin loaded Pluronic 85/lipid-polymeric nanoparticles for the treatment of leukemia: In vitro and in vivo therapeutic effect evaluation.Biomed Pharmacother. 2017 Feb;86:547-554. doi: 10.1016/j.biopha.2016.11.121. Epub 2016 Dec 23. Biomed Pharmacother. 2017. PMID: 28024291
-
Tumor-targeted and pH-controlled delivery of doxorubicin using gold nanorods for lung cancer therapy.Int J Nanomedicine. 2015 Oct 29;10:6773-88. doi: 10.2147/IJN.S93237. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26604751 Free PMC article.
-
Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment.Oncol Rep. 2017 Feb;37(2):1253-1260. doi: 10.3892/or.2017.5345. Epub 2017 Jan 2. Oncol Rep. 2017. PMID: 28075466
-
Multifunctional PLGA nanoparticles combining transferrin-targetability and pH-stimuli sensitivity enhanced doxorubicin intracellular delivery and in vitro antineoplastic activity in MDR tumor cells.Toxicol In Vitro. 2021 Sep;75:105192. doi: 10.1016/j.tiv.2021.105192. Epub 2021 May 11. Toxicol In Vitro. 2021. PMID: 33984456
Cited by
-
Comprehensive comparison of theranostic nanoparticles in breast cancer.Am J Clin Exp Immunol. 2022 Feb 15;11(1):1-27. eCollection 2022. Am J Clin Exp Immunol. 2022. PMID: 35350450 Free PMC article. Review.
-
Developing Actively Targeted Nanoparticles to Fight Cancer: Focus on Italian Research.Pharmaceutics. 2021 Sep 22;13(10):1538. doi: 10.3390/pharmaceutics13101538. Pharmaceutics. 2021. PMID: 34683830 Free PMC article. Review.
-
Systematic Review of Cancer Targeting by Nanoparticles Revealed a Global Association between Accumulation in Tumors and Spleen.Int J Mol Sci. 2021 Dec 1;22(23):13011. doi: 10.3390/ijms222313011. Int J Mol Sci. 2021. PMID: 34884816 Free PMC article.
-
Targeted Cancer Therapy via pH-Functionalized Nanoparticles: A Scoping Review of Methods and Outcomes.Gels. 2022 Apr 11;8(4):232. doi: 10.3390/gels8040232. Gels. 2022. PMID: 35448133 Free PMC article. Review.
-
Transferrin-Decorated PLGA Nanoparticles Loaded with an Organoselenium Compound as an Innovative Approach to Sensitize MDR Tumor Cells: An In Vitro Study Using 2D and 3D Cell Models.Nanomaterials (Basel). 2023 Aug 11;13(16):2306. doi: 10.3390/nano13162306. Nanomaterials (Basel). 2023. PMID: 37630891 Free PMC article.
References
-
- Le Q.-V., Choi J., Oh Y.-K. Nano delivery systems and cancer immunotherapy. J. Pharm. Investig. 2018;48:527–539. doi: 10.1007/s40005-018-0399-z. - DOI
-
- Gupta B., Yong C.S., Kim J.O. Solid matrix-based lipid nanoplatforms as carriers for combinational therapeutics in cancer. J. Pharm. Investig. 2017;47:461–473. doi: 10.1007/s40005-017-0337-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

