SUMOylation of Vps34 by SUMO1 promotes phenotypic switching of vascular smooth muscle cells by activating autophagy in pulmonary arterial hypertension
- PMID: 30703554
- PMCID: PMC6814199
- DOI: 10.1016/j.pupt.2019.01.007
SUMOylation of Vps34 by SUMO1 promotes phenotypic switching of vascular smooth muscle cells by activating autophagy in pulmonary arterial hypertension
Abstract
Introduction: Pulmonary arterial hypertension (PAH) is a life-threatening disease without effective therapies. PAH is associated with a progressive increase in pulmonary vascular resistance and irreversible pulmonary vascular remodeling. SUMO1 (small ubiquitin-related modifier 1) can bind to target proteins and lead to protein SUMOylation, an important post-translational modification with a key role in many diseases. However, the contribution of SUMO1 to PAH remains to be fully characterized.
Methods: In this study, we explored the role of SUMO1 in the dedifferentiation of vascular smooth muscle cells (VSMCs) involved in hypoxia-induced pulmonary vascular remodeling and PAH in vivo and in vitro.
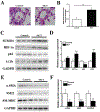
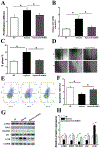
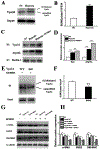
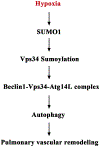
Results: In a mouse model of hypoxic PAH, SUMO1 expression was significantly increased, which was associated with activation of autophagy (increased LC3b and decreased p62), dedifferentiation of pulmonary arterial VSMCs (reduced α-SMA, SM22 and SM-MHC), and pulmonary vascular remodeling. Similar results were obtained in a MCT-induced PAH model. Overexpression of SUMO1 significantly increased VSMCs proliferation, migration, hypoxia-induced VSMCs dedifferentiation, and autophagy, but these effects were abolished by inhibition of autophagy by 3-MA in aortic VSMCs. Furthermore, SUMO1 knockdown reversed hypoxia-induced proliferation and migration of PASMCs. Mechanistically, SUMO1 promotes Vps34 SUMOylation and the assembly of the Beclin-1-Vps34-Atg14 complex, thereby inducing autophagy, whereas Vps34 mutation K840R reduces Vps34 SUMOylation and inhibits VSMCs dedifferentiation.
Discussion: Our data uncovers an important role of SUMO1 in VSMCs proliferation, migration, autophagy, and phenotypic switching (dedifferentiation) involved in pulmonary vascular remodeling and PAH. Targeting of the SUMO1-Vps34-autophagy signaling axis may be exploited to develop therapeutic strategies to treat PAH.
Keywords: Atg14; Beclin-1; Vps34.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

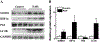
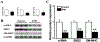


Similar articles
-
TMEM16A ameliorates vascular remodeling by suppressing autophagy via inhibiting Bcl-2-p62 complex formation.Theranostics. 2020 Mar 4;10(9):3980-3993. doi: 10.7150/thno.41028. eCollection 2020. Theranostics. 2020. PMID: 32226533 Free PMC article.
-
FGF12 (Fibroblast Growth Factor 12) Inhibits Vascular Smooth Muscle Cell Remodeling in Pulmonary Arterial Hypertension.Hypertension. 2020 Dec;76(6):1778-1786. doi: 10.1161/HYPERTENSIONAHA.120.15068. Epub 2020 Oct 26. Hypertension. 2020. PMID: 33100045
-
Nur77 downregulation triggers pulmonary artery smooth muscle cell proliferation and migration in mice with hypoxic pulmonary hypertension via the Axin2-β-catenin signaling pathway.Vascul Pharmacol. 2016 Dec;87:230-241. doi: 10.1016/j.vph.2016.11.002. Epub 2016 Nov 15. Vascul Pharmacol. 2016. PMID: 27871853
-
Vascular smooth muscle remodeling in health and disease.Can J Physiol Pharmacol. 2021 Feb;99(2):171-178. doi: 10.1139/cjpp-2020-0399. Epub 2020 Aug 27. Can J Physiol Pharmacol. 2021. PMID: 32853532 Review.
-
Regulatory Mechanisms Governing the Autophagy-Initiating VPS34 Complex and Its inhibitors.Biomol Ther (Seoul). 2024 Nov 1;32(6):723-735. doi: 10.4062/biomolther.2024.094. Epub 2024 Oct 7. Biomol Ther (Seoul). 2024. PMID: 39370737 Free PMC article. Review.
Cited by
-
Irisin suppresses PDGF-BB-induced proliferation of vascular smooth muscle cells in vitro by activating AMPK/mTOR-mediated autophagy.Eur J Histochem. 2024 Oct 15;68(4):4104. doi: 10.4081/ejh.2024.4104. Eur J Histochem. 2024. PMID: 39410813 Free PMC article.
-
Research progress on the mechanism of phenotypic transformation of pulmonary artery smooth muscle cells induced by hypoxia.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Dec 25;51(6):750-757. doi: 10.3724/zdxbyxb-2022-0282. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36915980 Free PMC article. Review. English.
-
DNA methylation in peripheral blood is associated with renal aging and renal function decline: a national community study.Clin Epigenetics. 2024 Jun 15;16(1):80. doi: 10.1186/s13148-024-01694-y. Clin Epigenetics. 2024. PMID: 38879526 Free PMC article.
-
Angiogenic factor AGGF1 acts as a tumor suppressor by modulating p53 post-transcriptional modifications and stability via MDM2.Cancer Lett. 2021 Jan 28;497:28-40. doi: 10.1016/j.canlet.2020.10.014. Epub 2020 Oct 15. Cancer Lett. 2021. PMID: 33069768 Free PMC article.
-
Astragaloside IV attenuates hypoxia‑induced pulmonary vascular remodeling via the Notch signaling pathway.Mol Med Rep. 2021 Jan;23(1):89. doi: 10.3892/mmr.2020.11726. Epub 2020 Nov 25. Mol Med Rep. 2021. PMID: 33236156 Free PMC article.
References
-
- Stenmark KR, Fagan KA, Frid MG, Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms, Circ. Res 99 (2006) 675–691. - PubMed
-
- Guignabert C, Dorfmuller P, Pathology and pathobiology of pulmonary hypertension, Semin. Respir. Crit. Care Med 38 (2017) 571–584. - PubMed
-
- Zhang W, Zhu T, Wu W, Ge X, Xiong X, Zhang Z, et al., LOX-1 mediated phenotypic switching of pulmonary arterial smooth muscle cells contributes to hypoxic pulmonary hypertension, Eur. J. Pharmacol 818 (2017) 84–95. - PubMed
-
- Zhu B, Gong Y, Yan G, Wang D, Qiao Y, Wang Q, et al., Down-regulation of lncRNA MEG3 promotes hypoxia-induced human pulmonary artery smooth muscle cell proliferation and migration via repressing PTEN by sponging miR-21, Biochem. Biophys. Res. Commun (2017) 2125–2132. - PubMed
-
- Yi B, Cui J, Ning JN, Wang GS, Qian GS, Lu KZ, Over-expression of PKGIalpha inhibits hypoxia-induced proliferation, Akt activation, and phenotype modulation of human PASMCs: the role of phenotype modulation of PASMCs in pulmonary vascular remodeling, Gene 492 (2012) 354–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

