A high-throughput microscopy method for single-cell analysis of event-time correlations in nanoparticle-induced cell death
- PMID: 30701200
- PMCID: PMC6345847
- DOI: 10.1038/s42003-019-0282-0
A high-throughput microscopy method for single-cell analysis of event-time correlations in nanoparticle-induced cell death
Abstract
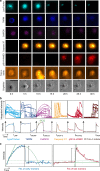
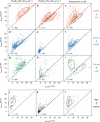
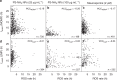
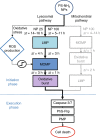
The temporal context of cell death decisions remains generally hidden in ensemble measurements with endpoint readouts. Here, we describe a method to extract event times from fluorescence time traces of cell death-related markers in automated live-cell imaging on single-cell arrays (LISCA) using epithelial A549 lung and Huh7 liver cancer cells as a model system. In pairwise marker combinations, we assess the chronological sequence and delay times of the events lysosomal membrane permeabilization, mitochondrial outer membrane permeabilization and oxidative burst after exposure to 58 nm amino-functionalized polystyrene nanoparticles (PS-NH2 nanoparticles). From two-dimensional event-time scatter plots we infer a lysosomal signal pathway at a low dose of nanoparticles (25 µg mL-1) for both cell lines, while at a higher dose (100 µg mL-1) a mitochondrial pathway coexists in A549 cells, but not in Huh7. In general, event-time correlations provide detailed insights into heterogeneity and interdependencies in signal transmission pathways.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A high-throughput real-time in vitro assay using mitochondrial targeted roGFP for screening of drugs targeting mitochondria.Redox Biol. 2019 Jan;20:379-389. doi: 10.1016/j.redox.2018.10.013. Epub 2018 Oct 24. Redox Biol. 2019. PMID: 30408753 Free PMC article.
-
The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes.Nanomedicine. 2013 Nov;9(8):1159-68. doi: 10.1016/j.nano.2013.04.010. Epub 2013 May 7. Nanomedicine. 2013. PMID: 23660460
-
Mitophagy-lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells.Ecotoxicol Environ Saf. 2021 Jan 15;208:111463. doi: 10.1016/j.ecoenv.2020.111463. Epub 2020 Oct 29. Ecotoxicol Environ Saf. 2021. PMID: 33130480
-
Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles.Open Biol. 2018 Apr;8(4):170271. doi: 10.1098/rsob.170271. Open Biol. 2018. PMID: 29643148 Free PMC article.
-
Lysosomal membrane permeabilization in cell death.Oncogene. 2008 Oct 27;27(50):6434-51. doi: 10.1038/onc.2008.310. Oncogene. 2008. PMID: 18955971 Review.
Cited by
-
Contribution of Particle-Induced Lysosomal Membrane Hyperpolarization to Lysosomal Membrane Permeabilization.Int J Mol Sci. 2021 Feb 25;22(5):2277. doi: 10.3390/ijms22052277. Int J Mol Sci. 2021. PMID: 33668885 Free PMC article.
-
Live imaging of apoptotic signaling flow using tunable combinatorial FRET-based bioprobes for cell population analysis of caspase cascades.Sci Rep. 2022 Dec 7;12(1):21160. doi: 10.1038/s41598-022-25286-z. Sci Rep. 2022. PMID: 36476686 Free PMC article.
-
An automated real-time microfluidic platform to probe single NK cell heterogeneity and cytotoxicity on-chip.Sci Rep. 2021 Aug 24;11(1):17084. doi: 10.1038/s41598-021-96609-9. Sci Rep. 2021. PMID: 34429486 Free PMC article.
-
Sequential and Switchable Patterning for Studying Cellular Processes under Spatiotemporal Control.ACS Appl Mater Interfaces. 2021 Aug 4;13(30):35545-35560. doi: 10.1021/acsami.1c09850. Epub 2021 Jul 20. ACS Appl Mater Interfaces. 2021. PMID: 34283577 Free PMC article.
-
High-Throughput Single-Cell Analysis of Nanoparticle-Cell Interactions.Trends Analyt Chem. 2023 Sep;166:117172. doi: 10.1016/j.trac.2023.117172. Epub 2023 Jul 10. Trends Analyt Chem. 2023. PMID: 37520860 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

