A Simplified Genomic Profiling Approach Predicts Outcome in Metastatic Colorectal Cancer
- PMID: 30691222
- PMCID: PMC6406354
- DOI: 10.3390/cancers11020147
A Simplified Genomic Profiling Approach Predicts Outcome in Metastatic Colorectal Cancer
Abstract
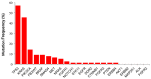
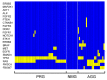
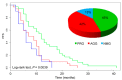
The response of metastatic colorectal cancer (mCRC) to the first-line conventional combination therapy is highly variable, reflecting the elevated heterogeneity of the disease. The genetic alterations underlying this heterogeneity have been thoroughly characterized through omic approaches requiring elevated efforts and costs. In order to translate the knowledge of CRC molecular heterogeneity into a practical clinical approach, we utilized a simplified Next Generation Sequencing (NGS) based platform to screen a cohort of 77 patients treated with first-line conventional therapy. Samples were sequenced using a panel of hotspots and targeted regions of 22 genes commonly involved in CRC. This revealed 51 patients carrying actionable gene mutations, 22 of which carried druggable alterations. These mutations were frequently associated with additional genetic alterations. To take into account this molecular complexity and assisted by an unbiased bioinformatic analysis, we defined three subgroups of patients carrying distinct molecular patterns. We demonstrated these three molecular subgroups are associated with a different response to first-line conventional combination therapies. The best outcome was achieved in patients exclusively carrying mutations on TP53 and/or RAS genes. By contrast, in patients carrying mutations in any of the other genes, alone or associated with mutations of TP53/RAS, the expected response is much worse compared to patients with exclusive TP53/RAS mutations. Additionally, our data indicate that the standard approach has limited efficacy in patients without any mutations in the genes included in the panel. In conclusion, we identified a reliable and easy-to-use approach for a simplified molecular-based stratification of mCRC patients that predicts the efficacy of the first-line conventional combination therapy.
Keywords: NGS; chemotherapy; genomic profiling; precision medicine; predictive.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
Similar articles
-
Clinical Multigene Panel Sequencing Identifies Distinct Mutational Association Patterns in Metastatic Colorectal Cancer.Front Oncol. 2020 May 7;10:560. doi: 10.3389/fonc.2020.00560. eCollection 2020. Front Oncol. 2020. PMID: 32457828 Free PMC article.
-
TP53 DNA Binding Domain Mutations Predict Progression-Free Survival of Bevacizumab Therapy in Metastatic Colorectal Cancer.Cancers (Basel). 2019 Jul 30;11(8):1079. doi: 10.3390/cancers11081079. Cancers (Basel). 2019. PMID: 31366114 Free PMC article.
-
Rare RAS Mutations in Metastatic Colorectal Cancer Detected During Routine RAS Genotyping Using Next Generation Sequencing.Target Oncol. 2016 Jun;11(3):363-70. doi: 10.1007/s11523-015-0404-7. Target Oncol. 2016. PMID: 26661077
-
From tumour heterogeneity to advances in precision treatment of colorectal cancer.Nat Rev Clin Oncol. 2017 Apr;14(4):235-246. doi: 10.1038/nrclinonc.2016.171. Epub 2016 Dec 6. Nat Rev Clin Oncol. 2017. PMID: 27922044 Review.
-
A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance.Cancer Gene Ther. 2015 Sep;22(9):417-30. doi: 10.1038/cgt.2015.39. Epub 2015 Sep 11. Cancer Gene Ther. 2015. PMID: 26358176 Review.
Cited by
-
An integrative in-silico analysis discloses a novel molecular subset of colorectal cancer possibly eligible for immune checkpoint immunotherapy.Biol Direct. 2022 May 9;17(1):10. doi: 10.1186/s13062-022-00324-y. Biol Direct. 2022. PMID: 35534873 Free PMC article.
-
Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation.Cell Death Dis. 2020 Dec 10;11(12):1045. doi: 10.1038/s41419-020-03174-6. Cell Death Dis. 2020. PMID: 33303756 Free PMC article.
-
Clinical Multigene Panel Sequencing Identifies Distinct Mutational Association Patterns in Metastatic Colorectal Cancer.Front Oncol. 2020 May 7;10:560. doi: 10.3389/fonc.2020.00560. eCollection 2020. Front Oncol. 2020. PMID: 32457828 Free PMC article.
-
Efficacy, safety and genomic analysis of SCT200, an anti-EGFR monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type metastatic colorectal cancer: a phase Ⅱ study.EBioMedicine. 2024 Feb;100:104966. doi: 10.1016/j.ebiom.2024.104966. Epub 2024 Jan 13. EBioMedicine. 2024. PMID: 38217945 Free PMC article. Clinical Trial.
-
Precision medicine for gastrointestinal cancer: Recent progress and future perspective.World J Gastrointest Oncol. 2020 Jan 15;12(1):1-20. doi: 10.4251/wjgo.v12.i1.1. World J Gastrointest Oncol. 2020. PMID: 31966910 Free PMC article. Review.
References
-
- Colucci G., Gebbia V., Paoletti G., Giuliani F., Caruso M., Gebbia N., Carteni G., Agostara B., Pezzella G., Manzione L., et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J. Clin. Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. - DOI - PubMed
-
- Tournigand C., Andre T., Achille E., Lledo G., Flesh M., Mery-Mignard D., Quinaux E., Couteau C., Buyse M., Ganem G., et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

