Different Transcriptomic Responses to Thermal Stress in Heat-Tolerant and Heat-Sensitive Pacific Abalones Indicated by Cardiac Performance
- PMID: 30687115
- PMCID: PMC6334008
- DOI: 10.3389/fphys.2018.01895
Different Transcriptomic Responses to Thermal Stress in Heat-Tolerant and Heat-Sensitive Pacific Abalones Indicated by Cardiac Performance
Abstract
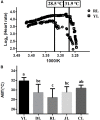
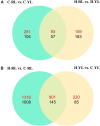
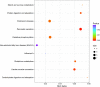
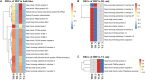
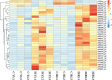
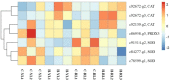
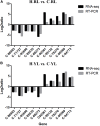
The Pacific abalone Haliotis discus hannai is one of the most economically important mollusks in China. Even though it has been farmed in southern China for almost 20 years, summer mortality remains the most challengeable problem for Pacific abalone aquaculture recently. Here, we determined the different heat tolerance ability for five selective lines of H. discus hannai by measuring the cardiac performance and Arrhenius breakpoint temperature (ABT). The Red line (RL) and Yangxia line (YL) were determined as the most heat-sensitive and most heat-tolerant line, respectively. Heart rates for RL were significantly lower than those of the YL at the same temperature (p < 0.05). The differentially expressed genes (DEGs), which were enriched in several pathways including cardiac muscle contraction, glutathione metabolism and oxidative phosphorylation, were identified between RL and YL at control temperature (20°C) and heat stress temperature (28.5°C, the ABT of the RL) by RNA-seq method. In the RL, 3370 DEGs were identified between the control and the heat-stress temperature, while only 1351 DEGs were identified in YL between these two temperature tests. Most of these DEGs were enriched in the pathways such as protein processing in endoplasmic reticulum, nucleotide binding and oligomerization domain (NOD) like receptor signaling, and ubiquitin mediated proteolysis. Notably, the most heat-tolerant line YL used an effective heat-protection strategy based on moderate transcriptional changes and regulation on the expression of key genes.
Keywords: ABT; Pacific abalone; cardiac performance; heat stress; transcriptome.
Figures



Similar articles
-
Transcriptomic responses to thermal stress in hybrid abalone (Haliotis discus hannai ♀ × H. fulgens ♂).Front Genet. 2022 Nov 16;13:1053674. doi: 10.3389/fgene.2022.1053674. eCollection 2022. Front Genet. 2022. PMID: 36467994 Free PMC article.
-
Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone.BMC Genomics. 2021 Sep 8;22(1):650. doi: 10.1186/s12864-021-07954-y. BMC Genomics. 2021. PMID: 34496767 Free PMC article.
-
Comparative Transcriptome and DNA Methylation Analysis of Phenotypic Plasticity in the Pacific Abalone (Haliotis discus hannai).Front Physiol. 2021 Jun 29;12:683499. doi: 10.3389/fphys.2021.683499. eCollection 2021. Front Physiol. 2021. PMID: 34267674 Free PMC article.
-
Expression of Heat Shock Proteins in Thermally Challenged Pacific Abalone Haliotis discus hannai.Genes (Basel). 2019 Dec 23;11(1):22. doi: 10.3390/genes11010022. Genes (Basel). 2019. PMID: 31878084 Free PMC article.
-
Transcriptome expression profiles between diploid and triploid Pacific abalone (Haliotis discus hannai) juveniles in response to acute heat-stress and hypoxia treatments.Mar Genomics. 2021 Jun;57:100820. doi: 10.1016/j.margen.2020.100820. Epub 2020 Oct 1. Mar Genomics. 2021. PMID: 33867117
Cited by
-
Transcriptomic responses to thermal stress in hybrid abalone (Haliotis discus hannai ♀ × H. fulgens ♂).Front Genet. 2022 Nov 16;13:1053674. doi: 10.3389/fgene.2022.1053674. eCollection 2022. Front Genet. 2022. PMID: 36467994 Free PMC article.
-
Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone.BMC Genomics. 2021 Sep 8;22(1):650. doi: 10.1186/s12864-021-07954-y. BMC Genomics. 2021. PMID: 34496767 Free PMC article.
-
Comparative Transcriptome and DNA Methylation Analysis of Phenotypic Plasticity in the Pacific Abalone (Haliotis discus hannai).Front Physiol. 2021 Jun 29;12:683499. doi: 10.3389/fphys.2021.683499. eCollection 2021. Front Physiol. 2021. PMID: 34267674 Free PMC article.
-
Expression of Heat Shock Proteins in Thermally Challenged Pacific Abalone Haliotis discus hannai.Genes (Basel). 2019 Dec 23;11(1):22. doi: 10.3390/genes11010022. Genes (Basel). 2019. PMID: 31878084 Free PMC article.
-
Curcumin Changed the Number, Particle Size, and miRNA Profile of Serum Exosomes in Roman Laying Hens under Heat Stress.Genes (Basel). 2024 Feb 8;15(2):217. doi: 10.3390/genes15020217. Genes (Basel). 2024. PMID: 38397207 Free PMC article.
References
-
- Abele D., Heise K., Portner H. O., Puntarulo S. (2002). Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 205 1831–1841. - PubMed
-
- Alter K., Andrewartha S. J., Morash A. J., Clark T. D., Hellicar A. D., Leon R. I., et al. (2017). Hybrid abalone are more robust to multi-stressor environments than pure parental species. Aquaculture 478 25–34. 10.1016/j.aquaculture.2017.04.035 - DOI
-
- Anders S., Huber W. (2012). Differential Expression of RNA-Seq Data at the Gene Level–the DESeq Package. Heidelberg: European Molecular Biology Laboratory (EMBL).
LinkOut - more resources
Full Text Sources

