Altered pharmacokinetics of combined oral contraceptives in obesity - multistudy assessment
- PMID: 30684471
- PMCID: PMC6441376
- DOI: 10.1016/j.contraception.2018.12.009
Altered pharmacokinetics of combined oral contraceptives in obesity - multistudy assessment
Abstract
Objective: The objective was to evaluate the pharmacokinetics (PKs) of levonorgestrel (LNG)-containing combined oral contraceptives (COCs) in obese women.
Study design: We pooled and reanalyzed data from 89 women with different body mass index (BMI) categories from four clinical studies. The LNG and ethinyl estradiol (EE) PKs were analyzed utilizing a zero-order absorption (K0), two-compartment PK model to evaluate key PK parameters in relation to a range of weights, BMI and body surface area (BSA).
Results: Increasing of body habitus metrics is correlated with decreasing Cmax (p<.0001) and AUCτ (p<.05) for both LNG and EE, but no correlation was found for Cmin (p≥.17). Increasing weight and BMI were associated with a modest increase (p≤.056) of clearance (CL) and appreciable increases of central volume (V1, p<.05), distribution clearance (CLd, p≤.001) and peripheral volume (V2, p<.0001) for LNG. For EE, increases in CL (p≤.009) were found with greater weight, BMI and BSA. Values of V1, CLd and V2 also increased (p<.0001) in obese subjects. The half-life and steady-state volume were greater among obese women (p<.0001) for both LNG and EE. LNG and EE PK parameters correlated well (p≤.006 for all), indicating that individual subject physiology affected both drugs similarly.
Conclusions: The primary effects of obesity on LNG and EE were a modest increase in CL and a marked increase in distribution parameters. We observed no obesity-related differences in trough LNG and EE concentrations.
Implications: This population PK analysis demonstrated reduced systemic exposure to LNG/EE oral contraceptives in obese subjects (Cmax and AUCτ); these particular differences are unlikely to lower contraceptive effectiveness among obese women who are correctly using LNG-containing contraceptives.
Keywords: Clearance; Combined oral contraceptives; Obesity; Pharmacokinetics; Volume of distribution.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
William J Jusko has been a recent consultant for Novartis, Boehringer Ingelheim, Reveragen, and Bayer Healthcare Products. A. Edelman: consultant for World Health Organization, Gynuity Health Projects, Genzyme, Agile Therapeutics, and HRAPharma. Nexplanon trainer for Merck and the recipient of a Merck-investigator initiated grant. Author for UptoDate (Royalties received). C Westhoff: consultant for Merck and Bayer, Agile Therapeutics. No conflicts of interest to disclose for the other authors.
Figures




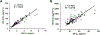
Similar articles
-
Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users.Contraception. 2013 Feb;87(2):220-6. doi: 10.1016/j.contraception.2012.10.008. Epub 2012 Nov 12. Contraception. 2013. PMID: 23153898 Free PMC article.
-
Effects of ritonavir-boosted protease inhibitors on combined oral contraceptive pharmacokinetics and pharmacodynamics in HIV-positive women.Contraception. 2019 Oct;100(4):283-287. doi: 10.1016/j.contraception.2019.06.002. Epub 2019 Jun 10. Contraception. 2019. PMID: 31194965 Free PMC article. Clinical Trial.
-
Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum.Contraception. 1994 Dec;50(6):563-79. doi: 10.1016/0010-7824(94)90014-0. Contraception. 1994. PMID: 7705098 Clinical Trial.
-
Perspectives on variability in pharmacokinetics of an oral contraceptive product.Contraception. 2017 Jan;95(1):5-9. doi: 10.1016/j.contraception.2016.07.019. Epub 2016 Jul 27. Contraception. 2017. PMID: 27475034 Free PMC article. Review.
-
Pharmacokinetics of gestagens: some problems.Am J Obstet Gynecol. 1990 Jul;163(1 Pt 2):323-8. doi: 10.1016/0002-9378(90)90576-s. Am J Obstet Gynecol. 1990. PMID: 2115297 Review.
Cited by
-
Contraception for Adolescents and Young Women with Type 2 Diabetes-Specific Considerations.Curr Diab Rep. 2022 Feb;22(2):77-84. doi: 10.1007/s11892-022-01448-1. Epub 2022 Feb 12. Curr Diab Rep. 2022. PMID: 35150410 Review.
-
Pharmacokinetics of Hormonal Contraception in Individuals with Obesity: a Review.Curr Obstet Gynecol Rep. 2020 Jun;9(2):72-78. doi: 10.1007/s13669-020-00284-y. Epub 2020 May 4. Curr Obstet Gynecol Rep. 2020. PMID: 33117601 Free PMC article.
-
Contraceptive Options Following Gestational Diabetes: Current Perspectives.Open Access J Contracept. 2019 Oct 22;10:41-53. doi: 10.2147/OAJC.S184821. eCollection 2019. Open Access J Contracept. 2019. PMID: 31749639 Free PMC article.
-
Levonorgestrel emergency contraception and bodyweight: are current recommendations consistent with historic data?J Drug Assess. 2020 Feb 10;9(1):37-42. doi: 10.1080/21556660.2020.1725524. eCollection 2020. J Drug Assess. 2020. PMID: 32166043 Free PMC article.
-
Phase 1 randomized trials to assess safety, pharmacokinetics, and vaginal bleeding associated with use of extended duration dapivirine and levonorgestrel vaginal rings.PLoS One. 2024 Jun 5;19(6):e0304552. doi: 10.1371/journal.pone.0304552. eCollection 2024. PLoS One. 2024. PMID: 38838028 Free PMC article. Clinical Trial.
References
-
- Centers for Disease Control and Prevention. http://wwwcdcgov/nchs/hus/contents2013.
-
- Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004:1–36. - PubMed
-
- Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2004;9:57–68. - PubMed
-
- Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46–52. - PubMed
-
- Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

