Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer's disease
- PMID: 30684442
- PMCID: PMC6366989
- DOI: 10.18632/aging.101759
Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer's disease
Abstract
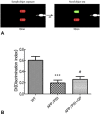
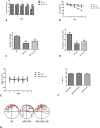
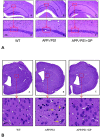
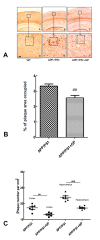
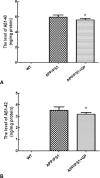
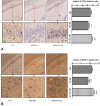
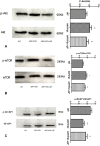
Geniposide, an iridoid glycoside extract from the gardenia fruit, is used in traditional Chinese medicine to alleviate symptoms of liver and inflammatory diseases. Geniposide activates GLP-1 receptors, known to modulate the activity of mechanistic target of rapamycin (mTOR), a key kinase regulating energy balance, proliferation, and survival in cells. mTOR activation inhibits autophagy, which is often disrupted in age-related diseases. Modulation of mTOR function to increase autophagy and inhibit apoptosis is involved in the protective effects of pharmacologic agents targeting diabetes and Alzheimer's disease (AD). We investigated whether such mechanism could mediate geniposide's neuroprotective effects in the APP/PS1 mouse model of AD. Eight-week treatment with geniposide improved cognitive scores in behavioral tests, reduced amyloid-β 1-40 plaque deposition, and reduced soluble Aβ1-40 and Aβ1-42 levels in the APP/PS1 mouse brain.This also showed increased p-Akt/Akt, p-mTOR/mTOR and decreased p-4E-BP1/4E-BP1 expression, and these patterns were partially reversed by geniposide. Evidence for enhanced autophagy, denoted by increased expression of LC3-II and Beclin1, was also seen after treatment with geniposide. Our data suggests that down regulation of mTOR signaling, leading to enhanced autophagy and lysosomal clearance of Aβ fibrils, underlies the beneficial effects of geniposide against neuropathological damage and cognitive deficits characteristic of AD.
Keywords: APP/PS1 mice; Alzheimer’s disease; autophagy; geniposide; mechanistic target of rapamycin.
Conflict of interest statement
Figures
Similar articles
-
Geniposide effectively reverses cognitive impairment and inhibits pathological cerebral damage by regulating the mTOR Signal pathway in APP∕PS1 mice.Neurosci Lett. 2020 Feb 16;720:134749. doi: 10.1016/j.neulet.2020.134749. Epub 2020 Jan 11. Neurosci Lett. 2020. PMID: 31935433
-
Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice.Eur J Pharmacol. 2014 Oct 5;740:312-20. doi: 10.1016/j.ejphar.2014.06.051. Epub 2014 Jul 17. Eur J Pharmacol. 2014. PMID: 25041840
-
Geniposide protection against Aβ1-42 toxicity correlates with mTOR inhibition and enhancement of autophagy.J Integr Neurosci. 2021 Mar 30;20(1):67-75. doi: 10.31083/j.jin.2021.01.242. J Integr Neurosci. 2021. PMID: 33834692
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
-
Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer's disease?J Neurosci Res. 2012 Jun;90(6):1105-18. doi: 10.1002/jnr.23011. Epub 2012 Feb 16. J Neurosci Res. 2012. PMID: 22344941 Review.
Cited by
-
Geniposide for treating atherosclerotic cardiovascular disease: a systematic review on its biological characteristics, pharmacology, pharmacokinetics, and toxicology.Chin Med. 2024 Aug 20;19(1):111. doi: 10.1186/s13020-024-00981-3. Chin Med. 2024. PMID: 39164773 Free PMC article. Review.
-
GLP-1/GIP Agonist as an Intriguing and Ultimate Remedy for Combating Alzheimer's Disease through its Supporting DPP4 Inhibitors: A Review.Curr Top Med Chem. 2024;24(19):1635-1664. doi: 10.2174/0115680266293416240515075450. Curr Top Med Chem. 2024. PMID: 38803170 Review.
-
The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer's disease.Front Endocrinol (Lausanne). 2022 Nov 17;13:1033479. doi: 10.3389/fendo.2022.1033479. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36465634 Free PMC article. Review.
-
Analysis of long noncoding RNA-associated competing endogenous RNA network in glucagon-like peptide-1 receptor agonist-mediated protection in β cells.World J Diabetes. 2020 Sep 15;11(9):374-390. doi: 10.4239/wjd.v11.i9.374. World J Diabetes. 2020. PMID: 32994866 Free PMC article.
-
The functional mechanism of bone marrow-derived mesenchymal stem cells in the treatment of animal models with Alzheimer's disease: crosstalk between autophagy and apoptosis.Stem Cell Res Ther. 2022 Mar 3;13(1):90. doi: 10.1186/s13287-022-02765-8. Stem Cell Res Ther. 2022. PMID: 35241159 Free PMC article. Review.
References
-
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;4:e9979. doi: 10.1371/journal.pone.0009979. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

