Cytomegalovirus viral load parameters associated with earlier initiation of pre-emptive therapy after solid organ transplantation
- PMID: 30682032
- PMCID: PMC6347177
- DOI: 10.1371/journal.pone.0210420
Cytomegalovirus viral load parameters associated with earlier initiation of pre-emptive therapy after solid organ transplantation
Abstract
Background: Human cytomegalovirus (HCMV) can be managed by monitoring HCMV DNA in the blood and giving valganciclovir when viral load exceeds a defined value. We hypothesised that such pre-emptive therapy should occur earlier than the standard 3000 genomes/ml (2520 IU/ml) when a seropositive donor transmitted virus to a seronegative recipient (D+R-) following solid organ transplantation (SOT).
Methods: Our local protocol was changed so that D+R- SOT patients commenced valganciclovir once the viral load exceeded 200 genomes/ml; 168 IU/ml (new protocol). The decision point remained at 3000 genomes/ml (old protocol) for the other two patient subgroups (D+R+, D-R+). Virological outcomes were assessed three years later, when 74 D+R- patients treated under the old protocol could be compared with 67 treated afterwards. The primary outcomes were changes in peak viral load, duration of viraemia and duration of treatment in the D+R- group. The secondary outcome was the proportion of D+R- patients who developed subsequent viraemia episodes.
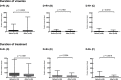
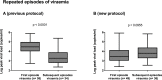
Findings: In the D+R- patients, the median values of peak viral load (30,774 to 11,135 genomes/ml, p<0.0215) were significantly reduced on the new protocol compared to the old, but the duration of viraemia and duration of treatment were not. Early treatment increased subsequent episodes of viraemia from 33/58 (57%) to 36/49 (73%) of patients (p< 0.0743) with a significant increase (p = 0.0072) in those episodes that required treatment (16/58; 27% versus 26/49; 53%). Median peak viral load increased significantly (2,103 to 3,934 genomes/ml, p<0.0249) in the D+R+ but not in the D-R+ patient subgroups. There was no change in duration of viraemia or duration of treatment for any patient subgroup.
Interpretation: Pre-emptive therapy initiated at the first sign of viraemia post-transplant significantly reduced the peak viral load but increased later episodes of viraemia, consistent with the hypothesis of reduced antigenic stimulation of the immune system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Cytomegalovirus pre-emptive therapy after hematopoietic stem cell transplantation in the era of real-time quantitative PCR: comparison with recipients of solid organ transplants.Transpl Infect Dis. 2016 Jun;18(3):405-14. doi: 10.1111/tid.12542. Epub 2016 Jun 9. Transpl Infect Dis. 2016. PMID: 27061703
-
Renal transplantation using kidneys from a donor with high grade cytomegalovirus viraemia: case report and literature review.Lancet Infect Dis. 2024 Nov;24(11):e718-e723. doi: 10.1016/S1473-3099(24)00359-1. Epub 2024 Jul 8. Lancet Infect Dis. 2024. PMID: 38991589 Review.
-
Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts.Liver Transpl. 2008 Feb;14(2):240-4. doi: 10.1002/lt.21362. Liver Transpl. 2008. PMID: 18236404 Clinical Trial.
-
Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients.Am J Transplant. 2010 Jan;10(1):157-61. doi: 10.1111/j.1600-6143.2009.02861.x. Epub 2009 Nov 4. Am J Transplant. 2010. PMID: 19889123
-
New developments in the management of cytomegalovirus infection after solid organ transplantation.Drugs. 2010 May 28;70(8):965-81. doi: 10.2165/10898540-000000000-00000. Drugs. 2010. PMID: 20481654 Review.
Cited by
-
Assessing Anti-HCMV Cell Mediated Immune Responses in Transplant Recipients and Healthy Controls Using a Novel Functional Assay.Front Cell Infect Microbiol. 2020 Jun 26;10:275. doi: 10.3389/fcimb.2020.00275. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670891 Free PMC article.
-
Are the Patterns of Cytomegalovirus Viral Load Seen After Solid Organ Transplantation Affected by Circadian Rhythm?J Infect Dis. 2022 Aug 24;226(2):357-365. doi: 10.1093/infdis/jiac055. J Infect Dis. 2022. PMID: 35184187 Free PMC article.
-
A Systematic Review and Meta-analysis of Optimized CMV Preemptive Therapy and Antiviral Prophylaxis for CMV Disease Prevention in CMV High-Risk (D+R-) Kidney Transplant Recipients.Transplant Direct. 2023 Jul 12;9(8):e1514. doi: 10.1097/TXD.0000000000001514. eCollection 2023 Aug. Transplant Direct. 2023. PMID: 37456587 Free PMC article.
-
A practical guide to real-world implementation of pre-emptive therapy for Cytomegalovirus disease prevention in high-risk seronegative liver transplant recipients with seropositive donors.Transpl Infect Dis. 2024 Jun;26(3):e14229. doi: 10.1111/tid.14229. Epub 2024 Jan 12. Transpl Infect Dis. 2024. PMID: 38214192
-
Unexpected Cytomegalovirus (CMV) Replication Kinetics in CMV Donor-Seropositive, Recipient-Seronegative Liver Transplant Recipients Receiving Preemptive Antiviral Therapy.J Infect Dis. 2022 Feb 1;225(3):436-442. doi: 10.1093/infdis/jiab132. J Infect Dis. 2022. PMID: 33755176 Free PMC article.
References
-
- Cope AV, Sabin C, Burroughs A, Rolles K, Griffiths PD, Emery VC. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. JInfectDis. 1997;176(6):1484–90. - PubMed
-
- Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The Efficacy and Safety of 200 Days Valganciclovir Cytomegalovirus Prophylaxis in High-Risk Kidney Transplant Recipients. AmJ Transplant. 2010;10(5):1228–37. - PubMed

