Cochlear compound action potentials from high-level tone bursts originate from wide cochlear regions that are offset toward the most sensitive cochlear region
- PMID: 30673362
- PMCID: PMC6520629
- DOI: 10.1152/jn.00677.2018
Cochlear compound action potentials from high-level tone bursts originate from wide cochlear regions that are offset toward the most sensitive cochlear region
Abstract
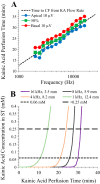
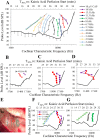
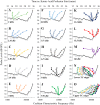
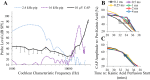
Little is known about the spatial origins of auditory nerve (AN) compound action potentials (CAPs) evoked by moderate to intense sounds. We studied the spatial origins of AN CAPs evoked by 2- to 16-kHz tone bursts at several sound levels by slowly injecting kainic acid solution into the cochlear apex of anesthetized guinea pigs. As the solution flowed from apex to base, it sequentially reduced CAP responses from low- to high-frequency cochlear regions. The times at which CAPs were reduced, combined with the cochlear location traversed by the solution at that time, showed the cochlear origin of the removed CAP component. For low-level tone bursts, the CAP origin along the cochlea was centered at the characteristic frequency (CF). As sound level increased, the CAP center shifted basally for low-frequency tone bursts but apically for high-frequency tone bursts. The apical shift was surprising because it is opposite the shift expected from AN tuning curve and basilar membrane motion asymmetries. For almost all high-level tone bursts, CAP spatial origins extended over 2 octaves along the cochlea. Surprisingly, CAPs evoked by high-level low-frequency (including 2 kHz) tone bursts showed little CAP contribution from CF regions ≤ 2 kHz. Our results can be mostly explained by spectral splatter from the tone-burst rise times, excitation in AN tuning-curve "tails," and asynchronous AN responses to high-level energy ≤ 2 kHz. This is the first time CAP origins have been identified by a spatially specific technique. Our results show the need for revising the interpretation of the cochlear origins of high-level CAPs-ABR wave 1. NEW & NOTEWORTHY Cochlear compound action potentials (CAPs) and auditory brain stem responses (ABRs) are routinely used in laboratories and clinics. They are typically interpreted as arising from the cochlear region tuned to the stimulus frequency. However, as sound level is increased, the cochlear origins of CAPs from tone bursts of all frequencies become very wide and their centers shift toward the most sensitive cochlear region. The standard interpretation of CAPs and ABRs from moderate to intense stimuli needs revision.
Keywords: auditory brain stem response; cochlear action potential; electrocochleography; kainic acid; synaptopathy.
Conflict of interest statement
C. A. Buchman has equity interest in Advanced Cochlear Diagnostics and is a consultant for Advanced Bionics Corporation, Med El Corporation, and Cochlear Corporation. Research projects in C. A. Buchman’s laboratory have been funded by Advanced Bionics Corporation, Med El Corporation, and Cochlear Corporation. A. N. Salt is a paid consultant to Otonomy, Inc., Cochlear Corporation, and Tusker Medical, Inc. Research projects in A. N. Salt’s laboratory have been funded by Cochlear Corporation, Frequency Therapeutics, Inc., and Hoffmann La Roche Pharmaceuticals.
Figures

Similar articles
-
A Guinea Pig Model Suggests That Objective Assessment of Acoustic Hearing Preservation in Human Ears With Cochlear Implants Is Confounded by Shifts in the Spatial Origin of Acoustically Evoked Potential Measurements Along the Cochlear Length.Ear Hear. 2024 May-Jun 01;45(3):666-678. doi: 10.1097/AUD.0000000000001457. Epub 2024 Jan 5. Ear Hear. 2024. PMID: 38178312
-
High-synchrony cochlear compound action potentials evoked by rising frequency-swept tone bursts.J Acoust Soc Am. 1985 Oct;78(4):1286-95. doi: 10.1121/1.392898. J Acoust Soc Am. 1985. PMID: 3840500
-
Acoustically Evoked Compound Action Potentials Recorded From Cochlear Implant Users With Preserved Acoustic Hearing.Ear Hear. 2023 Sep-Oct 01;44(5):1061-1077. doi: 10.1097/AUD.0000000000001350. Epub 2023 Aug 17. Ear Hear. 2023. PMID: 36882917 Free PMC article.
-
Mechanics of the mammalian cochlea.Physiol Rev. 2001 Jul;81(3):1305-52. doi: 10.1152/physrev.2001.81.3.1305. Physiol Rev. 2001. PMID: 11427697 Free PMC article. Review.
-
Mass and Stiffness Impact on the Middle Ear and the Cochlear Partition.J Audiol Otol. 2015 Apr;19(1):1-6. doi: 10.7874/jao.2015.19.1.1. Epub 2015 Apr 17. J Audiol Otol. 2015. PMID: 26185784 Free PMC article. Review.
Cited by
-
Early Detection of Endolymphatic Hydrops using the Auditory Nerve Overlapped Waveform (ANOW).Neuroscience. 2020 Jan 15;425:251-266. doi: 10.1016/j.neuroscience.2019.11.004. Epub 2019 Dec 3. Neuroscience. 2020. PMID: 31809731 Free PMC article.
-
The Spatial Origins of Cochlear Amplification Assessed by Stimulus-Frequency Otoacoustic Emissions.Biophys J. 2020 Mar 10;118(5):1183-1195. doi: 10.1016/j.bpj.2019.12.031. Epub 2020 Jan 3. Biophys J. 2020. PMID: 31968228 Free PMC article.
-
The Origin Along the Cochlea of Otoacoustic Emissions Evoked by Mid-Frequency Tone Pips.J Assoc Res Otolaryngol. 2024 Aug;25(4):363-376. doi: 10.1007/s10162-024-00955-0. Epub 2024 Jun 27. J Assoc Res Otolaryngol. 2024. PMID: 38937327
-
Optogenetics Reveals Roles for Supporting Cells in Force Transmission to and From Outer Hair Cells in the Mouse Cochlea.J Neurosci. 2024 Jan 24;44(4):e1179232023. doi: 10.1523/JNEUROSCI.1179-23.2023. J Neurosci. 2024. PMID: 38050104 Free PMC article.
-
Histological Correlates of Auditory Nerve Injury from Kainic Acid in the Budgerigar (Melopsittacus undulatus).J Assoc Res Otolaryngol. 2023 Oct;24(5):473-485. doi: 10.1007/s10162-023-00910-5. Epub 2023 Oct 5. J Assoc Res Otolaryngol. 2023. PMID: 37798548 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

