Transition from a meiotic to a somatic-like DNA damage response during the pachytene stage in mouse meiosis
- PMID: 30668564
- PMCID: PMC6358097
- DOI: 10.1371/journal.pgen.1007439
Transition from a meiotic to a somatic-like DNA damage response during the pachytene stage in mouse meiosis
Abstract
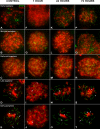
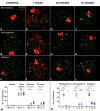
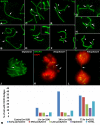
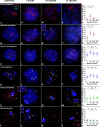
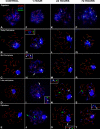
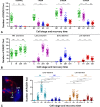
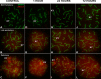
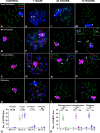
Homologous recombination (HR) is the principal mechanism of DNA repair acting during meiosis and is fundamental for the segregation of chromosomes and the increase of genetic diversity. Nevertheless, non-homologous end joining (NHEJ) mechanisms can also act during meiosis, mainly in response to exogenously-induced DNA damage in late stages of first meiotic prophase. In order to better understand the relationship between these two repair pathways, we studied the response to DNA damage during male mouse meiosis after gamma radiation. We clearly discerned two types of responses immediately after treatment. From leptotene to early pachytene, exogenous damage triggered the massive presence of γH2AX throughout the nucleus, which was associated with DNA repair mediated by HR components (DMC1 and RAD51). This early pathway finished with the sequential removal of DMC1 and RAD51 and was no longer inducible at mid pachytene. However, from mid-pachytene to diplotene, γH2AX appeared as large discrete foci. This late repair pattern was mediated initially by NHEJ, involving Ku70 and XRCC4, which were constitutively present, and 53BP1, which appeared at sites of damage soon after irradiation. Nevertheless, 24 hours after irradiation, a HR pathway involving RAD51 but not DMC1 mostly replaced NHEJ. Additionally, we observed the occurrence of synaptonemal complex bridges between bivalents, most likely representing chromosome translocation events that may involve DMC1, RAD51 or 53BP1. Our results reinforce the idea that the early "meiotic" repair pathway that acts by default at the beginning of meiosis is replaced from mid-pachytene onwards by a "somatic-like" repair pattern. This shift might be important to resolve DNA damage (either endogenous or exogenous) that could not be repaired by the early meiotic mechanisms, for instance those in the sex chromosomes, which lack a homologous chromosome to repair with. This transition represents another layer of functional changes that occur in meiotic cells during mid pachytene, in addition to epigenetic reprograming, reactivation of transcription, changes in the gene expression profile and acquisition of competence to proceed to metaphase.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
COM-1 promotes homologous recombination during Caenorhabditis elegans meiosis by antagonizing Ku-mediated non-homologous end joining.PLoS Genet. 2013;9(2):e1003276. doi: 10.1371/journal.pgen.1003276. Epub 2013 Feb 7. PLoS Genet. 2013. PMID: 23408909 Free PMC article.
-
Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation.PLoS Genet. 2013;9(12):e1003978. doi: 10.1371/journal.pgen.1003978. Epub 2013 Dec 19. PLoS Genet. 2013. PMID: 24367271 Free PMC article.
-
Radiation dosimetry and repair kinetics of DNA damage foci in mouse pachytene spermatocyte and round spermatid stages.Mutagenesis. 2018 Sep 17;33(3):231-239. doi: 10.1093/mutage/gey007. Mutagenesis. 2018. PMID: 30239864
-
Recent advances in functional conservation and divergence of recombinase RAD51 and DMC1.Yi Chuan. 2022 May 20;44(5):398-413. doi: 10.16288/j.yczz.22-016. Yi Chuan. 2022. PMID: 35729697 Review.
-
Double-stranded DNA breaks and gene functions in recombination and meiosis.Cell Res. 2006 May;16(5):402-12. doi: 10.1038/sj.cr.7310052. Cell Res. 2006. PMID: 16699536 Review.
Cited by
-
The Organotypic Culture of Mouse Seminiferous Tubules as a Reliable Methodology for the Study of Meiosis In Vitro.Methods Mol Biol. 2024;2818:147-160. doi: 10.1007/978-1-0716-3906-1_9. Methods Mol Biol. 2024. PMID: 39126472
-
Meiotic DNA breaks drive multifaceted mutagenesis in the human germ line.Science. 2023 Dec;382(6674):eadh2531. doi: 10.1126/science.adh2531. Epub 2023 Dec 1. Science. 2023. PMID: 38033082 Free PMC article.
-
A truncating variant of RAD51B associated with primary ovarian insufficiency provides insights into its meiotic and somatic functions.Cell Death Differ. 2022 Dec;29(12):2347-2361. doi: 10.1038/s41418-022-01021-z. Epub 2022 May 27. Cell Death Differ. 2022. PMID: 35624308 Free PMC article.
-
A reporter mouse for in vivo detection of DNA damage in embryonic germ cells.Genesis. 2020 Aug;58(8):e23368. doi: 10.1002/dvg.23368. Epub 2020 Apr 28. Genesis. 2020. PMID: 32343484 Free PMC article.
-
Investigating the potential of X chromosome shredding for mouse genetic biocontrol.Sci Rep. 2024 Jun 12;14(1):13466. doi: 10.1038/s41598-024-63706-4. Sci Rep. 2024. PMID: 38866815 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

