Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: A hope for disease-preventive therapy
- PMID: 30666755
- PMCID: PMC6398881
- DOI: 10.1111/cas.13948
Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: A hope for disease-preventive therapy
Abstract
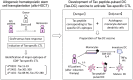
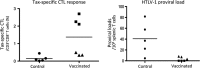
Adult T-cell leukemia/lymphoma (ATL) is an aggressive lymphoproliferative disease caused by human T-cell leukemia virus type 1 (HTLV-1). Multi-agent chemotherapy can reduce ATL cells but frequently allows relapses within a short period of time. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) following chemotherapy is now a standard therapy for ATL in Japan as it can achieve long-term remission in approximately one-third of recipient ATL patients; however, it also has a risk of treatment-related mortality. Allo-HSCT often induces HTLV-1 Tax-specific cytotoxic T cells (CTL) as well as graft-versus-host (GVH) response in ATL patients. This observation led to development of a new therapeutic vaccine to activate Tax-specific CTL, anticipating anti-ATL effects without GVH response. The newly developed Tax-DC vaccine consists of autologous dendritic cells pulsed with Tax peptides corresponding to CTL epitopes that have been identified in post-allo-HSCT ATL patients. In a pilot study of Tax-DC therapy in three ATL patients after various initial therapies, two patients survived for more than 4 years after vaccination without severe adverse effects (UMIN000011423). The Tax-DC vaccine is currently under phase I trial, showing a promising clinical outcome so far. These findings indicate the importance of patients' own HTLV-1-specific T-cell responses in maintaining remission and provide a new approach to anti-ATL immunotherapy targeting Tax. Although Tax-targeted vaccination is ineffective against Tax-negative ATL cells, it can be a safe alternative maintenance therapy for Tax-positive ATL and may be further applicable for treatment of indolent ATL or even prophylaxis of ATL development among HTLV-1-carriers.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest for this article.
Figures

Similar articles
-
Short-term cultured autologous peripheral blood mononuclear cells as a potential immunogen to activate Tax-specific CTL response in adult T-cell leukemia patients.Cancer Sci. 2021 Mar;112(3):1161-1172. doi: 10.1111/cas.14800. Epub 2021 Feb 5. Cancer Sci. 2021. PMID: 33410215 Free PMC article.
-
Long-term persistence of limited HTLV-I Tax-specific cytotoxic T cell clones in a patient with adult T cell leukemia/lymphoma after allogeneic stem cell transplantation.J Clin Immunol. 2012 Dec;32(6):1340-52. doi: 10.1007/s10875-012-9729-5. Epub 2012 Jul 5. J Clin Immunol. 2012. PMID: 22763862
-
Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study.Br J Haematol. 2015 May;169(3):356-67. doi: 10.1111/bjh.13302. Epub 2015 Jan 22. Br J Haematol. 2015. PMID: 25612920
-
Impact of host immunity on HTLV-1 pathogenesis: potential of Tax-targeted immunotherapy against ATL.Retrovirology. 2019 Aug 22;16(1):23. doi: 10.1186/s12977-019-0484-z. Retrovirology. 2019. PMID: 31438973 Free PMC article. Review.
-
Immunological risks of adult T-cell leukemia at primary HTLV-I infection.Trends Microbiol. 2004 Jul;12(7):346-52. doi: 10.1016/j.tim.2004.05.005. Trends Microbiol. 2004. PMID: 15223062 Review.
Cited by
-
Novel Treatments of Adult T Cell Leukemia Lymphoma.Front Microbiol. 2020 May 28;11:1062. doi: 10.3389/fmicb.2020.01062. eCollection 2020. Front Microbiol. 2020. PMID: 32547515 Free PMC article. Review.
-
Adult T-Cell Leukemia: a Comprehensive Overview on Current and Promising Treatment Modalities.Curr Oncol Rep. 2021 Nov 4;23(12):141. doi: 10.1007/s11912-021-01138-3. Curr Oncol Rep. 2021. PMID: 34735653 Review.
-
Tumor Antigens beyond the Human Exome.Int J Mol Sci. 2024 Apr 25;25(9):4673. doi: 10.3390/ijms25094673. Int J Mol Sci. 2024. PMID: 38731892 Free PMC article. Review.
-
Interferon regulatory factor 4 as a therapeutic target in adult T-cell leukemia lymphoma.Retrovirology. 2020 Aug 28;17(1):27. doi: 10.1186/s12977-020-00535-z. Retrovirology. 2020. PMID: 32859220 Free PMC article.
-
Efficacy of immune checkpoint inhibitor as maintenance therapy for advanced or metastatic cancers: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2022 Sep 23;101(38):e30830. doi: 10.1097/MD.0000000000030830. Medicine (Baltimore). 2022. PMID: 36197237 Free PMC article.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. - PubMed
-
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984‐87). Br J Haematol. 1991;79:428‐437. - PubMed
-
- Katsuya H, Shimokawa M, Ishitsuka K, et al. Prognostic index for chronic‐ and smoldering‐type adult T‐cell leukemia‐lymphoma. Blood. 2017;130:39‐47. - PubMed
-
- Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:15‐20. - PubMed
-
- Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T‐cell leukemia: a nationwide retrospective study. Blood. 2010;116:1369‐1376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

