CRISPR/Cas9 for overcoming drug resistance in solid tumors
- PMID: 30666557
- PMCID: PMC7214581
- DOI: 10.1007/s40199-019-00240-z
CRISPR/Cas9 for overcoming drug resistance in solid tumors
Abstract
Objectives: In this review, we focus on the application of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated nuclease 9 (Cas9), as a powerful genome editing system, in the identification of resistance mechanisms and in overcoming drug resistance in the most frequent solid tumors.
Data acquisition: Data were collected by conducting systematic searching of scientific English literature using specific keywords such as "cancer", "CRISPR" and related combinations.
Results: The review findings revealed the importance of CRISPR/Cas9 system in understanding drug resistance mechanisms and identification of resistance-related genes such as PBRM1, SLFN11 and ATPE1 in different cancers. We also provided an overview of genes, including RSF1, CDK5, and SGOL1, whose disruption can synergize with the currently available drugs such as paclitaxel and sorafenib.
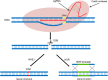
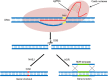
Conclusion: The data suggest CRISPR/Cas9 system as a useful tool in elucidating the molecular basis of drug resistance and improving clinical outcomes. Graphical abstract The mechanisms of CRISPR/Cas9-mediated genome editing and double-strand breaks (DSBs) repair.
Keywords: CRISPR/Cas9; Clinical outcome; Drug resistance; Drug response; Solid tumor; Targeted therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Modulating CRISPR/Cas9 genome-editing activity by small molecules.Drug Discov Today. 2022 Apr;27(4):951-966. doi: 10.1016/j.drudis.2021.11.018. Epub 2021 Nov 22. Drug Discov Today. 2022. PMID: 34823004 Review.
-
CRISPR/Cas9: a powerful tool for identification of new targets for cancer treatment.Drug Discov Today. 2019 Apr;24(4):955-970. doi: 10.1016/j.drudis.2019.02.011. Epub 2019 Mar 5. Drug Discov Today. 2019. PMID: 30849442 Review.
-
Hacking the Cancer Genome: Profiling Therapeutically Actionable Long Non-coding RNAs Using CRISPR-Cas9 Screening.Cancer Cell. 2019 Apr 15;35(4):545-557. doi: 10.1016/j.ccell.2019.01.019. Epub 2019 Feb 28. Cancer Cell. 2019. PMID: 30827888 Review.
-
Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy.Mol Cancer. 2023 Feb 16;22(1):35. doi: 10.1186/s12943-023-01738-6. Mol Cancer. 2023. PMID: 36797756 Free PMC article. Review.
-
Research into overcoming drug resistance in lung cancer treatment using CRISPR-Cas9 technology: a narrative review.Transl Lung Cancer Res. 2024 Aug 31;13(8):2067-2081. doi: 10.21037/tlcr-24-592. Epub 2024 Aug 28. Transl Lung Cancer Res. 2024. PMID: 39263032 Free PMC article. Review.
Cited by
-
Targeting Cancer with CRISPR/Cas9-Based Therapy.Int J Mol Sci. 2022 Jan 5;23(1):573. doi: 10.3390/ijms23010573. Int J Mol Sci. 2022. PMID: 35008996 Free PMC article. Review.
-
CX Chemokine Receptor 7 Contributes to Survival of KRAS-Mutant Non-Small Cell Lung Cancer upon Loss of Epidermal Growth Factor Receptor.Cancers (Basel). 2019 Mar 30;11(4):455. doi: 10.3390/cancers11040455. Cancers (Basel). 2019. PMID: 30935067 Free PMC article.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Biological Carriers for Drug Delivery in Cancer Therapy.Front Bioeng Biotechnol. 2022 Apr 14;10:882545. doi: 10.3389/fbioe.2022.882545. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35497332 Free PMC article. Review.
-
CRISPR-Cas9-based genome-wide screening identified novel targets for treating sorafenib-resistant hepatocellular carcinoma: a cross-talk between FGF21 and the NRF2 pathway.Sci China Life Sci. 2022 Oct;65(10):1998-2016. doi: 10.1007/s11427-021-2067-7. Epub 2022 Apr 1. Sci China Life Sci. 2022. PMID: 35380342
-
5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent.Front Oncol. 2021 Apr 19;11:658636. doi: 10.3389/fonc.2021.658636. eCollection 2021. Front Oncol. 2021. PMID: 33954114 Free PMC article. Review.
References
-
- van der Wekken AJ, Saber A, Hiltermann TJN, Kok K, van den Berg A, Groen HJM. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 2016;100:107–116. - PubMed
-
- Saber A, van der Wekken A, Hiltermann TJN, Kok K, van den Berg A, Groen HJM. Genomic aberrations guiding treatment of non-small cell lung cancer patients. Cancer Treat Commun. 2015;4:23–33.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

