Regulatory Roles of Sortilin and SorLA in Immune-Related Processes
- PMID: 30666202
- PMCID: PMC6330335
- DOI: 10.3389/fphar.2018.01507
Regulatory Roles of Sortilin and SorLA in Immune-Related Processes
Abstract
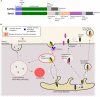
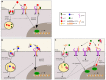
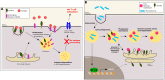
Sortilin, also known as Neurotensin Receptor-3, and the sorting-related receptor with type-A repeats (SorLA) are both members of the Vps10p domain receptor family. Initially identified in CNS cells, they are expressed in various other cell types where they exert multiple functions. Although mostly studied for its involvement in Alzheimer's disease, SorLA has recently been shown to be implicated in immune response by regulating IL-6-mediated signaling, as well as driving monocyte migration. Sortilin has been shown to act as a receptor, as a co-receptor and as an intra- and extracellular trafficking regulator. In the last two decades, deregulation of sortilin has been demonstrated to be involved in many human pathophysiologies, including neurodegenerative disorders (Alzheimer and Parkinson diseases), type 2 diabetes and obesity, cancer, and cardiovascular pathologies such as atherosclerosis. Several studies highlighted different functions of sortilin in the immune system, notably in microglia, pro-inflammatory cytokine regulation, phagosome fusion and pathogen clearance. In this review, we will analyze the multiple roles of sortilin and SorLA in the human immune system and how their deregulation may be involved in disease development.
Keywords: SorLA; cytokines; immune system; inflammation; phagocytosis; signaling; sortilin; trafficking.
Figures
Similar articles
-
Exploring the sortilin related receptor, SorLA, in depression.J Affect Disord. 2018 May;232:260-267. doi: 10.1016/j.jad.2018.02.050. Epub 2018 Feb 21. J Affect Disord. 2018. PMID: 29499509
-
The adaptor protein PICK1 targets the sorting receptor SorLA.Mol Brain. 2022 Feb 19;15(1):18. doi: 10.1186/s13041-022-00903-0. Mol Brain. 2022. PMID: 35183222 Free PMC article.
-
A new role under sortilin's belt in cancer.Commun Integr Biol. 2016 Jan 11;9(1):e1130192. doi: 10.1080/19420889.2015.1130192. eCollection 2016 Jan-Feb. Commun Integr Biol. 2016. PMID: 27066187 Free PMC article.
-
The implications of sortilin/vps10p domain receptors in neurological and human diseases.CNS Neurol Disord Drug Targets. 2014;13(8):1354-65. doi: 10.2174/1871527313666141023151642. CNS Neurol Disord Drug Targets. 2014. PMID: 25345507 Review.
-
Sortilin and SorLA regulate neuronal sorting of trophic and dementia-linked proteins.Mol Neurobiol. 2012 Apr;45(2):379-87. doi: 10.1007/s12035-012-8236-2. Mol Neurobiol. 2012. PMID: 22297619 Review.
Cited by
-
Insights into the regulatory role of epigenetics in moyamoya disease: Current advances and future prospectives.Mol Ther Nucleic Acids. 2024 Jul 19;35(3):102281. doi: 10.1016/j.omtn.2024.102281. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39188306 Free PMC article. Review.
-
Modulation of Small RNA Signatures in Schwann-Cell-Derived Extracellular Vesicles by the p75 Neurotrophin Receptor and Sortilin.Biomedicines. 2020 Oct 24;8(11):450. doi: 10.3390/biomedicines8110450. Biomedicines. 2020. PMID: 33114403 Free PMC article.
-
RUFY1 binds Arl8b and mediates endosome-to-TGN CI-M6PR retrieval for cargo sorting to lysosomes.J Cell Biol. 2023 Jan 2;222(1):e202108001. doi: 10.1083/jcb.202108001. Epub 2022 Oct 25. J Cell Biol. 2023. PMID: 36282215 Free PMC article.
-
Finding memo: versatile interactions of the VPS10p-Domain receptors in Alzheimer's disease.Mol Neurodegener. 2022 Nov 18;17(1):74. doi: 10.1186/s13024-022-00576-2. Mol Neurodegener. 2022. PMID: 36397124 Free PMC article. Review.
-
Sortilin is associated with progranulin deficiency and autism-like behaviors in valproic acid-induced autism rats.CNS Neurosci Ther. 2024 Sep;30(9):e70015. doi: 10.1111/cns.70015. CNS Neurosci Ther. 2024. PMID: 39218796 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

