Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture
- PMID: 30663158
- PMCID: PMC6496165
- DOI: 10.1111/cpr.12570
Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture
Abstract
Objectives: Exosomes, as important players in intercellular communication due to their ability to transfer certain molecules to target cells, are believed to take similar effects in promoting bone regeneration with their derived stem cells. Studies have suggested that umbilical cord mesenchymal stem cells (uMSCs) could promote angiogenesis. This study investigated whether exosomes derived from uMSCs (uMSC-Exos) could enhance fracture healing as primary factors by promoting angiogenesis.
Materials and methods: uMSCs were obtained to isolate uMSC-Exos by ultrafiltration, with exosomes from human embryonic kidney 293 cells (HEK293) and phosphate-buffered saline (PBS) being used as control groups. NanoSight, laser light scattering spectrometer, transmission electron microscopy and Western blotting were used to identify exosomes. Next, uMSC-Exos combined with hydrogel were transplanted into the fracture site in a rat model of femoral fracture. Bone healing processes were monitored and evaluated by radiographic methods on days 7, 14, 21 and 31 after surgery; angiogenesis of the fracture sites was assessed by radiographic and histological strategies on post-operative day 14. In vitro, the expression levels of osteogenesis- or angiogenesis-related genes after being cultured with uMSC-Exos were identified by qRT-PCR. The internalization ability of exosomes was determined using the PKH67 assay. Cell cycle analysis, EdU incorporation and immunofluorescence staining, scratch wound assay and tube formation analysis were also used to determine the altered abilities of human umbilical vein endothelial cells (HUVECs) administered with uMSC-Exos in proliferation, migration and angiogenesis. Finally, to further explore the underlying molecular mechanisms, specific RNA inhibitors or siRNAs were used, and the subsequent effects were observed.
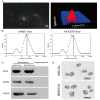
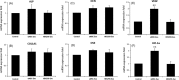
Results: uMSC-Exos had a diameter of approximately 100 nm, were spherical, meanwhile expressing CD9, CD63 and CD81. Transplantation of uMSC-Exos markedly enhanced angiogenesis and bone healing processes in a rat model of femoral fracture. In vitro, other than enhancing osteogenic differentiation, uMSC-Exos increased the expression of vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1α (HIF-1α). uMSC-Exos were taken up by HUVECs and enhanced their proliferation, migration and tube formation. Finally, by using specific RNA inhibitors or siRNAs, it has been confirmed that HIF-1α played an important role in the uMSC-Exos-induced VEGF expression, pro-angiogenesis and enhanced fracture repair, which may be one of the underlying mechanisms.
Conclusions: These results revealed a novel role of exosomes in uMSC-mediated therapy and suggested that implanted uMSC-Exos may represent a crucial clinical strategy to accelerate fracture healing via the promotion of angiogenesis. HIF-1α played an important role in this process.
Keywords: HIF-1α; angiogenesis; exosomes; fracture healing; umbilical cord mesenchymal stem cell.
© 2019 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures



Similar articles
-
Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion.Stem Cell Res Ther. 2020 Jan 28;11(1):38. doi: 10.1186/s13287-020-1562-9. Stem Cell Res Ther. 2020. PMID: 31992369 Free PMC article.
-
Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126.Acta Biomater. 2020 Feb;103:196-212. doi: 10.1016/j.actbio.2019.12.020. Epub 2019 Dec 17. Acta Biomater. 2020. PMID: 31857259
-
Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit.Cell Biol Int. 2017 Dec;41(12):1379-1390. doi: 10.1002/cbin.10869. Epub 2017 Sep 25. Cell Biol Int. 2017. PMID: 28877384
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
The potential therapeutic effect of human umbilical cord mesenchymal stem cell-derived exosomes in bronchopulmonary dysplasia.Life Sci. 2024 Nov 15;357:123047. doi: 10.1016/j.lfs.2024.123047. Epub 2024 Sep 12. Life Sci. 2024. PMID: 39260518 Review.
Cited by
-
ECFC-derived exosomal THBS1 mediates angiogenesis and osteogenesis in distraction osteogenesis via the PI3K/AKT/ERK pathway.J Orthop Translat. 2022 Sep 23;37:12-22. doi: 10.1016/j.jot.2022.08.004. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36196150 Free PMC article.
-
MicroRNA-21-3p Engineered Umbilical Cord Stem Cell-Derived Exosomes Inhibit Tendon Adhesion.J Inflamm Res. 2020 Jul 7;13:303-316. doi: 10.2147/JIR.S254879. eCollection 2020. J Inflamm Res. 2020. PMID: 32753931 Free PMC article.
-
Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing.J Nanobiotechnology. 2023 Jan 16;21(1):14. doi: 10.1186/s12951-023-01778-6. J Nanobiotechnology. 2023. PMID: 36642728 Free PMC article. Review.
-
Horizon of exosome-mediated bone tissue regeneration: The all-rounder role in biomaterial engineering.Mater Today Bio. 2022 Jul 11;16:100355. doi: 10.1016/j.mtbio.2022.100355. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 35875196 Free PMC article. Review.
-
Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review.Biomater Transl. 2021 Dec 28;2(4):343-360. doi: 10.12336/biomatertransl.2021.04.008. eCollection 2021. Biomater Transl. 2021. PMID: 35837417 Free PMC article. Review.
References
-
- Murata K, Ito H, Yoshitomi H, et al. Inhibition of miR‐92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J Bone Miner Res. 2014;29:316‐326. - PubMed
-
- Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385‐386. - PubMed
-
- Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505‐512. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

