The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins
- PMID: 30662405
- PMCID: PMC6328495
- DOI: 10.3389/fphar.2018.01515
The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins
Abstract
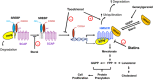
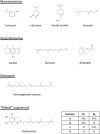
The mevalonate pathway provides sterols for membrane structure and nonsterol intermediates for the post-translational modification and membrane anchorage of growth-related proteins, including the Ras, Rac, and Rho GTPase family. Mevalonate-derived products are also essential for the Hedgehog pathway, steroid hormone signaling, and the nuclear localization of Yes-associated protein and transcriptional co-activator with PDZ-binding motif, all of which playing roles in tumorigenesis and cancer stem cell function. The phosphatidylinositol-4,5-bisphosphate 3-kinase-AKT-mammalian target of rapamycin complex 1 pathway, p53 with gain-of-function mutation, and oncoprotein MYC upregulate the mevalonate pathway, whereas adenosine monophosphate-activated protein kinase and tumor suppressor protein RB are the downregulators. The rate-limiting enzyme, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), is under a multivalent regulation. Sterol regulatory element binding protein 2 mediates the sterol-controlled transcriptional downregulation of HMGCR. UbiA prenyltransferase domain-containing protein-1 regulates the ubiquitination and proteasome-mediated degradation of HMGCR, which is accelerated by 24, 25-dihydrolanosterol and the diterpene geranylgeraniol. Statins, competitive inhibitors of HMGCR, deplete cells of mevalonate-derived intermediates and consequently inhibit cell proliferation and induce apoptosis. Clinical application of statins is marred by dose-limiting toxicities and mixed outcomes on cancer risk, survival and mortality, partially resulting from the statin-mediated compensatory upregulation of HMGCR and indiscriminate inhibition of HMGCR in normal and tumor cells. Tumor HMGCR is resistant to the sterol-mediated transcriptional control; consequently, HMGCR is upregulated in cancers derived from adrenal gland, blood and lymph, brain, breast, colon, connective tissue, embryo, esophagus, liver, lung, ovary, pancreas, prostate, skin, and stomach. Nevertheless, tumor HMGCR remains sensitive to isoprenoid-mediated degradation. Isoprenoids including monoterpenes (carvacrol, L-carvone, geraniol, perillyl alcohol), sesquiterpenes (cacalol, farnesol, β-ionone), diterpene (geranylgeranyl acetone), "mixed" isoprenoids (tocotrienols), and their derivatives suppress the growth of tumor cells with little impact on non-malignant cells. In cancer cells derived from breast, colon, liver, mesothelium, prostate, pancreas, and skin, statins and isoprenoids, including tocotrienols, geraniol, limonene, β-ionone and perillyl alcohol, synergistically suppress cell proliferation and associated signaling pathways. A blend of dietary lovastatin and δ-tocotrienol, each at no-effect doses, suppress the growth of implanted murine B16 melanomas in C57BL6 mice. Isoprenoids have potential as adjuvant agents to reduce the toxicities of statins in cancer prevention or therapy.
Keywords: HMG CoA reductase; SREBP; cancer; isoprenoids; mevalonate; statin; synergy.
Figures
Similar articles
-
Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention.Exp Biol Med (Maywood). 2004 Jul;229(7):567-85. doi: 10.1177/153537020422900701. Exp Biol Med (Maywood). 2004. PMID: 15229351 Review.
-
Synthesis, function, and regulation of sterol and nonsterol isoprenoids.Front Mol Biosci. 2022 Oct 5;9:1006822. doi: 10.3389/fmolb.2022.1006822. eCollection 2022. Front Mol Biosci. 2022. PMID: 36275615 Free PMC article. Review.
-
Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo.Exp Biol Med (Maywood). 2007 Apr;232(4):523-31. Exp Biol Med (Maywood). 2007. PMID: 17392488
-
Plant-derived monoterpenes suppress hamster kidney cell 3-hydroxy-3-methylglutaryl coenzyme a reductase synthesis at the post-transcriptional level.J Nutr. 2003 Jan;133(1):38-44. doi: 10.1093/jn/133.1.38. J Nutr. 2003. PMID: 12514264
-
Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer.Proc Soc Exp Biol Med. 1999 Sep;221(4):294-311. doi: 10.1046/j.1525-1373.1999.d01-87.x. Proc Soc Exp Biol Med. 1999. PMID: 10460692 Review.
Cited by
-
Lipid metabolism in pancreatic cancer: emerging roles and potential targets.Cancer Commun (Lond). 2022 Dec;42(12):1234-1256. doi: 10.1002/cac2.12360. Epub 2022 Sep 15. Cancer Commun (Lond). 2022. PMID: 36107801 Free PMC article. Review.
-
Lipid composition of the cancer cell membrane.J Bioenerg Biomembr. 2020 Oct;52(5):321-342. doi: 10.1007/s10863-020-09846-4. Epub 2020 Jul 26. J Bioenerg Biomembr. 2020. PMID: 32715369 Free PMC article. Review.
-
Anti-cancer mechanisms of linalool and 1,8-cineole in non-small cell lung cancer A549 cells.Heliyon. 2020 Dec 15;6(12):e05639. doi: 10.1016/j.heliyon.2020.e05639. eCollection 2020 Dec. Heliyon. 2020. PMID: 33367122 Free PMC article.
-
The Mevalonate Pathway, a Metabolic Target in Cancer Therapy.Front Oncol. 2021 Feb 25;11:626971. doi: 10.3389/fonc.2021.626971. eCollection 2021. Front Oncol. 2021. PMID: 33718197 Free PMC article. Review.
-
Bioactive lipids in cancer stem cells.World J Stem Cells. 2019 Sep 26;11(9):693-704. doi: 10.4252/wjsc.v11.i9.693. World J Stem Cells. 2019. PMID: 31616544 Free PMC article. Review.
References
-
- Alayoubi A. Y., Anderson J. F., Satyanarayanajois S. D., Sylvester P. W., Nazzal S. (2012). Concurrent delivery of tocotrienols and simvastatin by lipid nanoemulsions potentiates their antitumor activity against human mammary adenocarcenoma cells. Eur. J. Pharm. Sci. 48, 385–392. 10.1016/j.ejps.2012.12.011 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

