Impact of topical anti-fibrotics on corneal nerve regeneration in vivo
- PMID: 30660507
- PMCID: PMC6443430
- DOI: 10.1016/j.exer.2019.01.017
Impact of topical anti-fibrotics on corneal nerve regeneration in vivo
Abstract
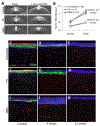
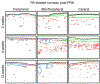
Recent work in vitro has shown that fibroblasts and myofibroblasts have opposing effects on neurite outgrowth by peripheral sensory neurons. Here, we tested a prediction from this work that dampening the fibrotic response in the early phases of corneal wound healing in vivo could enhance reinnervation after a large, deep corneal injury such as that induced by photorefractive keratectomy (PRK). Since topical steroids and Mitomycin C (MMC) are often used clinically for mitigating corneal inflammation and scarring after PRK, they were ideal to test this prediction. Twenty adult cats underwent bilateral, myopic PRK over a 6 mm optical zone followed by either: (1) intraoperative MMC (n = 12 eyes), (2) intraoperative prednisolone acetate (PA) followed by twice daily topical application for 14 days (n = 12 eyes), or (3) no post-operative treatment (n = 16 eyes). Anti-fibrotic effects of MMC and PA were verified optically and histologically. First, optical coherence tomography (OCT) performed pre-operatively and 2, 4 and 12 weeks post-PRK was used to assess changes in corneal backscatter reflectivity. Post-mortem immunohistochemistry was then performed at 2, 4 and 12 weeks post-PRK, using antibodies against α-smooth muscle actin (α-SMA). Finally, immunohistochemistry with antibodies against βIII-tubulin (Tuj-1) was performed in the same corneas to quantify changes in nerve distribution relative to unoperated, control cat corneas. Two weeks after PRK, untreated corneas exhibited the greatest amount of staining for α-SMA, followed by PA-treated and MMC-treated eyes. This was matched by higher OCT-based stromal reflectivity values in untreated, than PA- and MMC-treated eyes. PA treatment appeared to slow epithelial healing and although normal epithelial thickness was restored by 12 weeks-post-PRK, intra-epithelial nerve length only reached ∼1/6 normal values in PA-treated eyes. Even peripheral cornea (outside the ablation zone) exhibited depressed intra-epithelial nerve densities after PA treatment. Stromal nerves were abundant under the α-SMA zone, but appeared to largely avoid it, creating an area of sub-epithelial stroma devoid of nerve trunks. In turn, this may have led to the lack of sub-basal and intra-epithelial nerves in the ablation zone of PA-treated eyes 4 weeks after PRK, and their continuing paucity 12 weeks after PRK. Intra-operative MMC, which sharply decreased α-SMA staining, was followed by rapid restoration of nerve densities in all corneal layers post-PRK compared to untreated corneas. Curiously, stromal nerves appeared unaffected by the development of large, stromal, acellular zones in MMC-treated corneas. Overall, it appears that post-PRK treatments that were most effective at reducing α-SMA-positive cells in the early post-operative period benefited nerve regeneration the most, resulting in more rapid restoration of nerve densities in all corneal layers of the ablation zone and of the corneal periphery.
Keywords: Epithelium; Laser refractive surgery; Prednisolone acetate; Stroma; Sub-basal layer; Wound healing.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
There are no conflicts of interest for any of the authors.
Figures

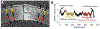



Similar articles
-
Mitomycin C-induced reduction of keratocytes and fibroblasts after photorefractive keratectomy.Invest Ophthalmol Vis Sci. 2004 Sep;45(9):2978-84. doi: 10.1167/iovs.04-0070. Invest Ophthalmol Vis Sci. 2004. PMID: 15326110
-
The Impact of Photorefractive Keratectomy and Mitomycin C on Corneal Nerves and Their Regeneration.J Refract Surg. 2018 Dec 1;34(12):790-798. doi: 10.3928/1081597X-20181112-01. J Refract Surg. 2018. PMID: 30540361 Free PMC article.
-
Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK.Curr Eye Res. 1998 Jul;17(7):736-47. Curr Eye Res. 1998. PMID: 9678420
-
Biological effects of mitomycin C on late corneal haze stromal fibrosis following PRK.Exp Eye Res. 2020 Nov;200:108218. doi: 10.1016/j.exer.2020.108218. Epub 2020 Sep 6. Exp Eye Res. 2020. PMID: 32905844 Free PMC article. Review.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
Cited by
-
A Novel CD147 Inhibitor, SP-8356, Attenuates Pathological Fibrosis in Alkali-Burned Rat Cornea.Int J Mol Sci. 2020 Apr 23;21(8):2990. doi: 10.3390/ijms21082990. Int J Mol Sci. 2020. PMID: 32340317 Free PMC article.
-
Blocking Mitochondrial Pyruvate Transport Alters Corneal Myofibroblast Phenotype: A New Target for Treating Fibrosis.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):36. doi: 10.1167/iovs.64.13.36. Invest Ophthalmol Vis Sci. 2023. PMID: 37870848 Free PMC article.
-
Biology of keratorefractive surgery- PRK, PTK, LASIK, SMILE, inlays and other refractive procedures.Exp Eye Res. 2020 Sep;198:108136. doi: 10.1016/j.exer.2020.108136. Epub 2020 Jul 10. Exp Eye Res. 2020. PMID: 32653492 Free PMC article. Review.
-
How scars shape the neural landscape: Key molecular mediators of TGF-β1's anti-neuritogenic effects.PLoS One. 2020 Nov 24;15(11):e0234950. doi: 10.1371/journal.pone.0234950. eCollection 2020. PLoS One. 2020. PMID: 33232327 Free PMC article.
-
Semaphorin 3A potentiates the profibrotic effects of transforming growth factor-β1 in the cornea.Biochem Biophys Res Commun. 2020 Jan 8;521(2):333-339. doi: 10.1016/j.bbrc.2019.10.107. Epub 2019 Oct 24. Biochem Biophys Res Commun. 2020. PMID: 31668808 Free PMC article.
References
-
- Araki K, Ohashi Y, Kinoshita S, Hayashi K, Kuwayama Y, Tano Y, 1994. Epithelial wound healing in the denervated cornea. Current Eye Research 13, 203–211. - PubMed
-
- Bahn CF, Meyer RF, MacCallum DK, Lillie JH, Lovett EJ, Sugar A, Martonyi CL, 1982. Penetrating keratoplasty in the cat. A clinically-applicable model. Ophthalmology 89, 687–699. - PubMed
-
- Bech F, Gonzalez-Gonzalez O, Artime E, Serrano J, Alcalde I, Gallar J, Merayo-Lloves J, Belmonte C, 2018. Functional and morphologic alterations in mechanical, polymodal, and cold sensory nerve fibers of the cornea following photorefractive keratectomy. Investigative Ophthalmolgy and Visual Science 59, 2281–2292. - PubMed
-
- Belmonte C, Acosta MC, Gallar J, 2004a. Neural basis of sensation in intact and injured corneas. Experimental Eye Research 78, 513–525. - PubMed
-
- Belmonte C, Acosta MC, Gallar J, 2004b. Neural basis of sensation in intact and injured corneas. Exp Eye Res 78, 513–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

