Cancer Immunotherapy: Silencing Intracellular Negative Immune Regulators of Dendritic Cells
- PMID: 30658461
- PMCID: PMC6357062
- DOI: 10.3390/cancers11010108
Cancer Immunotherapy: Silencing Intracellular Negative Immune Regulators of Dendritic Cells
Abstract
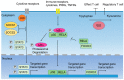
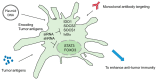
Dendritic cells (DCs) are capable of activating adaptive immune responses, or inducing immune suppression or tolerance. In the tumor microenvironment, the function of DCs is polarized into immune suppression that attenuates the effect of T cells, promoting differentiation of regulatory T cells and supporting tumor progression. Therefore, blocking negative immune regulators in DCs is considered a strategy of cancer immunotherapy. Antibodies can target molecules on the cell surface, but not intracellular molecules of DCs. The delivery of short-hairpin RNAs (shRNA) and small-interfering RNAs (siRNA) should be a strategy to silence specific intracellular targets in DCs. This review provides an overview of the known negative immune regulators of DCs. Moreover, a combination of shRNA/siRNA and DC vaccines, DNA vaccines in animal models, and clinical trials are also discussed.
Keywords: cancer; dendritic cells (DCs); intracellular negative immune regulator; short-hairpin RNA (shRNA); small-interfering RNA (siRNA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A new cancer immunotherapy via simultaneous DC-mobilization and DC-targeted IDO gene silencing using an immune-stimulatory nanosystem.Int J Cancer. 2018 Oct 15;143(8):2039-2052. doi: 10.1002/ijc.31588. Epub 2018 Aug 10. Int J Cancer. 2018. PMID: 29752722
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-β receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity.Clin Exp Immunol. 2015 Jul;181(1):164-78. doi: 10.1111/cei.12620. Epub 2015 May 17. Clin Exp Immunol. 2015. PMID: 25753156 Free PMC article.
-
Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies.Nanomedicine. 2010 Aug;6(4):523-9. doi: 10.1016/j.nano.2010.01.001. Epub 2010 Jan 18. Nanomedicine. 2010. PMID: 20085824 Review.
-
RNA transfer and its use in dendritic cell-based immunotherapy.Expert Opin Biol Ther. 2005 Feb;5(2):173-81. doi: 10.1517/14712598.5.2.173. Expert Opin Biol Ther. 2005. PMID: 15757379 Review.
Cited by
-
An innovative gene expression modulating strategy by converting nucleic acids into HNC therapeutics using carrier-free nanoparticles.Front Immunol. 2024 Jan 11;14:1343428. doi: 10.3389/fimmu.2023.1343428. eCollection 2023. Front Immunol. 2024. PMID: 38274829 Free PMC article.
-
Electrotransfer of siRNA to Silence Enhanced Green Fluorescent Protein in Tumor Mediated by a High Intensity Pulsed Electromagnetic Field.Vaccines (Basel). 2020 Jan 27;8(1):49. doi: 10.3390/vaccines8010049. Vaccines (Basel). 2020. PMID: 32012775 Free PMC article.
-
Cancer Vaccines: Research and Applications.Cancers (Basel). 2019 Jul 24;11(8):1041. doi: 10.3390/cancers11081041. Cancers (Basel). 2019. PMID: 31344788 Free PMC article.
References
-
- Diamond M.S., Kinder M., Matsushita H., Mashayekhi M., Dunn G.P., Archambault J.M., Lee H., Arthur C.D., White J.M., Kalinke U., et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

