Crystal Structures of Fumarate Hydratases from Leishmania major in a Complex with Inhibitor 2-Thiomalate
- PMID: 30645090
- PMCID: PMC6380369
- DOI: 10.1021/acschembio.8b00972
Crystal Structures of Fumarate Hydratases from Leishmania major in a Complex with Inhibitor 2-Thiomalate
Abstract
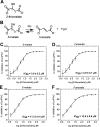
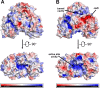
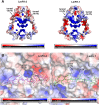
Leishmaniases affect the poorest people on earth and have no effective drug therapy. Here, we present the crystal structure of the mitochondrial isoform of class I fumarate hydratase (FH) from Leishmania major and compare it to the previously determined cytosolic Leishmania major isoform. We further describe the mechanism of action of the first class-specific FH inhibitor, 2-thiomalate, through X-ray crystallography and inhibition assays. Our crystal structures of both FH isoforms with inhibitor bound at 2.05 Å resolution and 1.60 Å resolution show high structural similarity. These structures further reveal that the selectivity of 2-thiomalate for class I FHs is due to direct coordination of the inhibitor to the unique Fe of the catalytic [4Fe-4S] cluster that is found in class I parasitic FHs but is absent from class II human FH. These studies provide the structural scaffold in order to exploit class I FHs as potential drug targets against leishmaniases as well as Chagas diseases, sleeping sickness, and malaria.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Structural and Biochemical Investigations of the [4Fe-4S] Cluster-Containing Fumarate Hydratase from Leishmania major.Biochemistry. 2019 Dec 10;58(49):5011-5021. doi: 10.1021/acs.biochem.9b00923. Epub 2019 Nov 27. Biochemistry. 2019. PMID: 31743022 Free PMC article.
-
Crystal structure of an Fe-S cluster-containing fumarate hydratase enzyme from Leishmania major reveals a unique protein fold.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):9804-9. doi: 10.1073/pnas.1605031113. Epub 2016 Aug 15. Proc Natl Acad Sci U S A. 2016. PMID: 27528683 Free PMC article.
-
Fumarate hydratase isoforms of Leishmania major: subcellular localization, structural and kinetic properties.Int J Biol Macromol. 2012 Jul-Aug;51(1-2):25-31. doi: 10.1016/j.ijbiomac.2012.04.025. Epub 2012 May 5. Int J Biol Macromol. 2012. PMID: 22569531
-
Crystal structure of dihydroorotate dehydrogenase from Leishmania major.Biochimie. 2012 Aug;94(8):1739-48. doi: 10.1016/j.biochi.2012.04.003. Epub 2012 Apr 21. Biochimie. 2012. PMID: 22542640
-
Antiparasitic chemotherapy: tinkering with the purine salvage pathway.Adv Exp Med Biol. 2008;625:116-32. doi: 10.1007/978-0-387-77570-8_10. Adv Exp Med Biol. 2008. PMID: 18365663 Review.
Cited by
-
Aurothiomalate-Based Drugs as Potentially Novel Agents Against Leishmania major: A Mini Review.Acta Parasitol. 2022 Jun;67(2):640-647. doi: 10.1007/s11686-022-00536-2. Epub 2022 Apr 5. Acta Parasitol. 2022. PMID: 35380401 Review.
-
Structural and Biochemical Investigations of the [4Fe-4S] Cluster-Containing Fumarate Hydratase from Leishmania major.Biochemistry. 2019 Dec 10;58(49):5011-5021. doi: 10.1021/acs.biochem.9b00923. Epub 2019 Nov 27. Biochemistry. 2019. PMID: 31743022 Free PMC article.
References
-
- Field M. C.; Horn D.; Fairlamb A. H.; Ferguson M. A. J.; Gray D. W.; Read K. D.; De Rycker M.; Torrie L. S.; Wyatt P. G.; Wyllie S.; Gilbert I. H. (2017) Anti-trypanosomatid drug discovery: an ongoing challenge and a continuing need. Nat. Rev. Microbiol. 15, 217.10.1038/nrmicro.2016.193. - DOI - PMC - PubMed
-
- Coustou V.; Besteiro S.; Riviere L.; Biran M.; Biteau N.; Franconi J. M.; Boshart M.; Baltz T.; Bringaud F. (2005) A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. J. Biol. Chem. 280, 16559–16570. 10.1074/jbc.M500343200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

