Sequential therapy with bevacizumab and EGFR inhibitors for metastatic colorectal carcinoma: a national registry-based analysis
- PMID: 30643461
- PMCID: PMC6314050
- DOI: 10.2147/CMAR.S183093
Sequential therapy with bevacizumab and EGFR inhibitors for metastatic colorectal carcinoma: a national registry-based analysis
Abstract
Purpose: Although inhibitors of vascular endothelial growth factor and inhibitors of epidermal growth factor receptor (EGFRi) are commonly used for the treatment of metastatic colorectal cancer (mCRC), the optimal sequencing of these agents is currently unclear.
Methods: A national registry of targeted therapies was used to analyze baseline characteristics and outcomes of patients with mCRC and wild-type KRAS exon 2 status who received bevacizumab and EGFRi (cetuximab or panitumumab) as a part of first- and second-line treatment in either sequence.
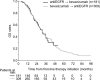
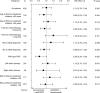
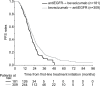
Results: The cohort included 490 patients (181 patients treated with first-line EGFRi and second-line bevacizumab and 309 patients treated with first-line bevacizumab and second-line EGFRi). Median overall survival (OS) from the initiation on first-line therapy was similar for patients treated with either sequence, reaching 31.8 (95% CI 27.5-36.1) vs 31.4 months (95% CI 27.8-35.0) for EGFRi → bevacizumab vs bevacizumab → EGFRi cohort, respectively. Time from first-line initiation to progression on the second-line therapy [progression-free survival (PFS)] was 21.1 (95% CI 19.3-23.0) vs 19.3 months (95% CI 17.3-21.3) for bevacizumab → EGFRi vs EGFRi → bevacizumab cohort, respectively (P=0.016).
Conclusion: This retrospective analysis of real-world data of patients with wild-type KRAS exon 2 mCRC showed no differences in OS between cohorts treated with bevacizumab → EGFRi vs the reverse sequence while combined PFS favored the bevacizumab → EGFRi sequence.
Keywords: bevacizumab; cetuximab; colorectal carcinoma; panitumumab; sequence.
Conflict of interest statement
Disclosure TB, OF, and BM have received research funding, travel grants, and honoraria from Roche, Merck, and Amgen. AP has received honoraria and travel grants from Roche and Amgen. IK has received speakers’ honoraria from Roche, Merck, and Amgen. JF has received lecture honoraria and travel grants from Roche, Merck, Pfizer, Novartis, and Amgen. The authors declare no other conflicts of interest in this work.
Figures

Similar articles
-
Evaluating the Optimal Sequence of Treatment With EGFR Inhibitors and Bevacizumab in RAS Wild-Type Metastatic Colorectal Cancer.Cureus. 2022 Mar 27;14(3):e23543. doi: 10.7759/cureus.23543. eCollection 2022 Mar. Cureus. 2022. PMID: 35494924 Free PMC article.
-
Exploratory pooled analysis evaluating the effect of sequence of biological therapies on overall survival in patients with RAS wild-type metastatic colorectal carcinoma.ESMO Open. 2018 Feb 24;3(2):e000297. doi: 10.1136/esmoopen-2017-000297. eCollection 2018. ESMO Open. 2018. PMID: 29531837 Free PMC article.
-
A study-level meta-analysis of efficacy data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in patients with RAS wild-type metastatic colorectal cancer.Eur J Cancer. 2016 Nov;67:11-20. doi: 10.1016/j.ejca.2016.07.019. Epub 2016 Sep 1. Eur J Cancer. 2016. PMID: 27592068
-
The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model.Health Technol Assess. 2013 Apr;17(14):1-237. doi: 10.3310/hta17140. Health Technol Assess. 2013. PMID: 23547747 Free PMC article. Review.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
Cited by
-
Sequential Treatment with Bevacizumab and Aflibercept for Metastatic Colorectal Cancer in Real-World Clinical Practice.Target Oncol. 2020 Apr;15(2):193-201. doi: 10.1007/s11523-020-00705-1. Target Oncol. 2020. PMID: 32052341
-
Characteristics and Absolute Survival of Metastatic Colorectal Cancer Patients Treated With Biologics: A Real-World Data Analysis From Three European Countries.Front Oncol. 2021 Mar 5;11:630456. doi: 10.3389/fonc.2021.630456. eCollection 2021. Front Oncol. 2021. PMID: 33747950 Free PMC article.
-
Regorafenib for Metastatic Colorectal Cancer: An Analysis of a Registry-Based Cohort of 555 Patients.Cancer Manag Res. 2020 Jul 3;12:5365-5372. doi: 10.2147/CMAR.S255332. eCollection 2020. Cancer Manag Res. 2020. PMID: 32753954 Free PMC article.
-
Evaluating the Optimal Sequence of Treatment With EGFR Inhibitors and Bevacizumab in RAS Wild-Type Metastatic Colorectal Cancer.Cureus. 2022 Mar 27;14(3):e23543. doi: 10.7759/cureus.23543. eCollection 2022 Mar. Cureus. 2022. PMID: 35494924 Free PMC article.
-
Optimal Sequence and Second-Line Systemic Treatment of Patients with RAS Wild-Type Metastatic Colorectal Cancer: A Meta-Analysis.J Clin Med. 2021 Nov 4;10(21):5166. doi: 10.3390/jcm10215166. J Clin Med. 2021. PMID: 34768686 Free PMC article.
References
-
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. - PubMed
-
- Rivera F, Karthaus M, Hecht JR, et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis. 2017;32(8):1179–1190. - PMC - PubMed
-
- Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. 2015;14(2):81–90. - PubMed
-
- Pietrantonio F, Cremolini C, Petrelli F, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;96(1):156–166. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

