Rates of hospitalization and death for all-cause and rotavirus acute gastroenteritis before rotavirus vaccine introduction in Kenya, 2010-2013
- PMID: 30634922
- PMCID: PMC6330491
- DOI: 10.1186/s12879-018-3615-6
Rates of hospitalization and death for all-cause and rotavirus acute gastroenteritis before rotavirus vaccine introduction in Kenya, 2010-2013
Abstract
Background: Rotavirus vaccine was introduced in Kenya immunization program in July 2014. Pre-vaccine disease burden estimates are important for assessing vaccine impact.
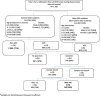
Methods: Children with acute gastroenteritis (AGE) (≥3 loose stools and/or ≥ 1 episode of unexplained vomiting followed by loose stool within a 24-h period), hospitalized in Siaya County Referral Hospital (SCRH) from January 2010 through December 2013 were enrolled. Stool specimens were tested for rotavirus (RV) using an enzyme immunoassay (EIA). Hospitalization rates were calculated using person-years of observation (PYO) from the Health Demographic Surveillance System (HDSS) as a denominator, while adjusting for healthcare utilization at household level and proportion of stool specimen collected from patients who met the case definition at the surveillance hospital. Mortality rates were calculated using PYO as the denominator and number of deaths estimated using total deaths in the HDSS, proportion of deaths attributed to diarrhoea by verbal autopsy (VA) and percent positive for rotavirus AGE (RVAGE) hospitalizations.
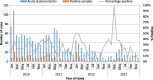
Results: Of 7760 all-cause hospitalizations among children < 5 years of age, 3793 (49%) were included in the analysis. Of these, 21% (805) had AGE; RV was detected in 143 (26%) of 541 stools tested. Among children < 5 years, the estimated hospitalization rates per 100,000 PYO for AGE and RVAGE were 2413 and 429, respectively. Mortality rate associated with AGE and RVAGE were 176 and 45 per 100,000 PYO, respectively.
Conclusion: AGE and RVAGE caused substantial health care burden (hospitalizations and deaths) before rotavirus vaccine introduction in Kenya.
Keywords: Children; Kenyan; Morbidity; Mortality; Rotavirus.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from all the guardians or caretakers of the children before enrolment into the study. This study was approved as part of the HDSS by both the Ethical Review Committee of the Kenya Medical Research Institute and CDC-Atlanta.
Consent for publication
Not applicable.
Competing interests
None declared.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
High burden of rotavirus gastroenteritis in young children in rural western Kenya, 2010-2011.Pediatr Infect Dis J. 2014 Jan;33 Suppl 1(Suppl 1):S34-40. doi: 10.1097/INF.0000000000000049. Pediatr Infect Dis J. 2014. PMID: 24343611 Free PMC article.
-
The epidemiology of all-cause and rotavirus acute gastroenteritis and the characteristics of rotavirus circulating strains before and after rotavirus vaccine introduction in Yemen: analysis of hospital-based surveillance data.BMC Infect Dis. 2015 Oct 13;15:418. doi: 10.1186/s12879-015-1165-8. BMC Infect Dis. 2015. PMID: 26464210 Free PMC article.
-
Distribution of rotavirus genotypes associated with acute diarrhoea in Zimbabwean children less than five years old before and after rotavirus vaccine introduction.Vaccine. 2018 Nov 12;36(47):7248-7255. doi: 10.1016/j.vaccine.2018.03.069. Epub 2018 Apr 5. Vaccine. 2018. PMID: 29628149
-
Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar.Vaccine. 2018 Nov 12;36(47):7198-7204. doi: 10.1016/j.vaccine.2017.08.091. Epub 2017 Sep 25. Vaccine. 2018. PMID: 28958809 Free PMC article.
-
Sustained impact of rotavirus vaccine introduction on rotavirus gastroenteritis hospitalizations in children <5 years of age, Ghana, 2009-2016.Vaccine. 2018 Nov 12;36(47):7131-7134. doi: 10.1016/j.vaccine.2018.02.058. Epub 2018 May 8. Vaccine. 2018. PMID: 29752020 Free PMC article.
Cited by
-
Epidemiology and pre-vaccine burden of rotavirus diarrhea in Democratic Republic of Congo (DRC): Results of sentinel surveillance, 2009-2019.Vaccine. 2022 Sep 29;40(41):5933-5941. doi: 10.1016/j.vaccine.2022.08.041. Epub 2022 Sep 6. Vaccine. 2022. PMID: 36068112 Free PMC article.
-
Report on advances for pediatricians in 2018: allergy, cardiology, critical care, endocrinology, hereditary metabolic diseases, gastroenterology, infectious diseases, neonatology, nutrition, respiratory tract disorders and surgery.Ital J Pediatr. 2019 Oct 16;45(1):126. doi: 10.1186/s13052-019-0727-6. Ital J Pediatr. 2019. PMID: 31619283 Free PMC article. Review.
-
Deployment of Rotavirus Vaccine in Western Kenya Coincides with a Reduction in All-Cause Child Mortality: A Retrospective Cohort Study.Vaccines (Basel). 2023 Jul 29;11(8):1299. doi: 10.3390/vaccines11081299. Vaccines (Basel). 2023. PMID: 37631867 Free PMC article.
-
Post-Discharge Risk of Mortality in Children under 5 Years of Age in Western Kenya: A Retrospective Cohort Study.Am J Trop Med Hyg. 2023 Aug 7;109(3):704-712. doi: 10.4269/ajtmh.23-0186. Print 2023 Sep 6. Am J Trop Med Hyg. 2023. PMID: 37549893 Free PMC article.
-
Effectiveness of rotavirus vaccine in the prevention of diarrhoeal diseases among children under age five years in Kavango East and West Regions, Namibia.J Public Health Afr. 2021 Jun 18;12(1):1680. doi: 10.4081/jphia.2021.1680. eCollection 2021 Jun 18. J Public Health Afr. 2021. PMID: 34249294 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

