Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency
- PMID: 30634580
- PMCID: PMC6356537
- DOI: 10.3390/cancers11010068
Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency
Abstract
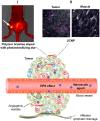
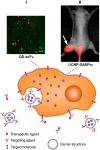
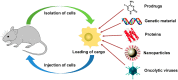
Malignant tumors are characterized by structural and molecular peculiarities providing a possibility to directionally deliver antitumor drugs with minimal impact on healthy tissues and reduced side effects. Newly formed blood vessels in malignant lesions exhibit chaotic growth, disordered structure, irregular shape and diameter, protrusions, and blind ends, resulting in immature vasculature; the newly formed lymphatic vessels also have aberrant structure. Structural features of the tumor vasculature determine relatively easy penetration of large molecules as well as nanometer-sized particles through a blood⁻tissue barrier and their accumulation in a tumor tissue. Also, malignant cells have altered molecular profile due to significant changes in tumor cell metabolism at every level from the genome to metabolome. Recently, the tumor interaction with cells of immune system becomes the focus of particular attention, that among others findings resulted in extensive study of cells with preferential tropism to tumor. In this review we summarize the information on the diversity of currently existing approaches to targeted drug delivery to tumor, including (i) passive targeting based on the specific features of tumor vasculature, (ii) active targeting which implies a specific binding of the antitumor agent with its molecular target, and (iii) cell-mediated tumor targeting.
Keywords: EPR-effect; active targeting; cancer treatment; cancer-specific molecular targets; cell-mediated targeting; passive targeting; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Macromolecular Drug Carriers for Targeted Glioblastoma Therapy: Preclinical Studies, Challenges, and Future Perspectives.Front Oncol. 2018 Dec 17;8:624. doi: 10.3389/fonc.2018.00624. eCollection 2018. Front Oncol. 2018. PMID: 30619758 Free PMC article. Review.
-
[The development of novel tumor targeting delivery strategy].Yao Xue Xue Bao. 2016 Feb;51(2):272-80. Yao Xue Xue Bao. 2016. PMID: 29856581 Review. Chinese.
-
Combination drug delivery via multilamellar vesicles enables targeting of tumor cells and tumor vasculature.Biotechnol Bioeng. 2018 Jun;115(6):1403-1415. doi: 10.1002/bit.26566. Epub 2018 Mar 6. Biotechnol Bioeng. 2018. PMID: 29457630
-
Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 nm Ultrafine Iron Oxide Nanoparticles.Theranostics. 2020 Jan 22;10(6):2479-2494. doi: 10.7150/thno.39560. eCollection 2020. Theranostics. 2020. PMID: 32194814 Free PMC article.
-
Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment.Int J Nanomedicine. 2019 Nov 1;14:8543-8560. doi: 10.2147/IJN.S219820. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31802868 Free PMC article.
Cited by
-
UCNP-based Photoluminescent Nanomedicines for Targeted Imaging and Theranostics of Cancer.Molecules. 2020 Sep 19;25(18):4302. doi: 10.3390/molecules25184302. Molecules. 2020. PMID: 32961731 Free PMC article.
-
Mechanisms of extracellular vesicle uptake and implications for the design of cancer therapeutics.J Extracell Biol. 2024 Oct 30;3(11):e70017. doi: 10.1002/jex2.70017. eCollection 2024 Nov. J Extracell Biol. 2024. PMID: 39483807 Free PMC article. Review.
-
Hydrogen Sulfide Responsive Phototherapy Agents: Design Strategies and Biological Applications.ACS Bio Med Chem Au. 2023 Jun 23;3(4):305-321. doi: 10.1021/acsbiomedchemau.3c00028. eCollection 2023 Aug 16. ACS Bio Med Chem Au. 2023. PMID: 37599789 Free PMC article. Review.
-
Soft and Condensed Nanoparticles and Nanoformulations for Cancer Drug Delivery and Repurpose.Adv Ther (Weinh). 2020 Jan;3(1):1900102. doi: 10.1002/adtp.201900102. Epub 2019 Oct 16. Adv Ther (Weinh). 2020. PMID: 34291146 Free PMC article.
-
Current approaches of nanomedicines in the market and various stage of clinical translation.Acta Pharm Sin B. 2022 Jul;12(7):3028-3048. doi: 10.1016/j.apsb.2022.02.025. Epub 2022 Mar 1. Acta Pharm Sin B. 2022. PMID: 35865096 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

