Genetic variation of kinases and activation of nucleotide analog reverse transcriptase inhibitor tenofovir
- PMID: 30628547
- PMCID: PMC6563221
- DOI: 10.2217/pgs-2018-0140
Genetic variation of kinases and activation of nucleotide analog reverse transcriptase inhibitor tenofovir
Abstract
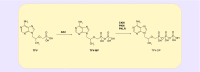
As antiretroviral therapy has become more accessible across the world and coformulations have improved patient compliance; the morbidity and mortality of HIV/AIDS has decreased. However, there is still a substantial gap in knowledge regarding the impact of genetic variation on the metabolism of and response to some of the most commonly prescribed antiretrovirals, including the nucleotide reverse transcriptase inhibitor tenofovir. While it has been scientifically established that tenofovir must be activated to be efficacious against HIV, the enzymes responsible for this activation have not been well characterized. The purpose of this review is to summarize and clarify the scientific knowledge regarding the enzymes that phosphorylate and activate this clinically important drug.
Keywords: HIV; antiretroviral therapy; drug metabolism; genetic variants; precision medicine.
Conflict of interest statement
This work was supported by NIH (grant numbers R01 AI128781, UM1 AI068613 and UM1 AI106707). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
Similar articles
-
Tenofovir or zidovudine in three-drug combination therapy with one nucleoside reverse transcriptase inhibitor and one non-nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD008740. doi: 10.1002/14651858.CD008740. Cochrane Database Syst Rev. 2010. PMID: 20927777 Review.
-
Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection.Clin Ther. 2002 Oct;24(10):1515-48. doi: 10.1016/s0149-2918(02)80058-3. Clin Ther. 2002. PMID: 12462284 Review.
-
[Clinical data II. Clinical experience of tenofovir DF in combination with protease inhibitors].Enferm Infecc Microbiol Clin. 2008 Jun;26 Suppl 8:13-8. doi: 10.1157/13126267. Enferm Infecc Microbiol Clin. 2008. PMID: 19195433 Review. Spanish.
-
[Clinical data I. Clinical experience with tenofovir in combination with nonnucleoside analogue transcriptase inhibitors].Enferm Infecc Microbiol Clin. 2008 Jun;26 Suppl 8:7-12. doi: 10.1157/13126266. Enferm Infecc Microbiol Clin. 2008. PMID: 19195432 Review. Spanish.
-
Body composition and metabolic outcomes after 96 weeks of treatment with ritonavir-boosted lopinavir plus either nucleoside or nucleotide reverse transcriptase inhibitors or raltegravir in patients with HIV with virological failure of a standard first-line antiretroviral therapy regimen: a substudy of the randomised, open-label, non-inferiority SECOND-LINE study.Lancet HIV. 2017 Jan;4(1):e13-e20. doi: 10.1016/S2352-3018(16)30189-8. Epub 2016 Nov 1. Lancet HIV. 2017. PMID: 27815068 Clinical Trial.
Cited by
-
Viral dissemination and immune activation modulate antiretroviral drug levels in lymph nodes of SIV-infected rhesus macaques.Front Immunol. 2023 Sep 18;14:1213455. doi: 10.3389/fimmu.2023.1213455. eCollection 2023. Front Immunol. 2023. PMID: 37790938 Free PMC article.
-
Effect of alcohol exposure on the efficacy and safety of tenofovir alafenamide fumarate, a major medicine against human immunodeficiency virus.Biochem Pharmacol. 2022 Oct;204:115224. doi: 10.1016/j.bcp.2022.115224. Epub 2022 Aug 22. Biochem Pharmacol. 2022. PMID: 36007574 Free PMC article.
-
An open-label, randomized, single intravenous dosing study to investigate the effect of fixed-dose combinations of tenofovir/lamivudine or atazanavir/ritonavir on the pharmacokinetics of remdesivir in Ugandan healthy volunteers (RemTLAR).Trials. 2021 Nov 23;22(1):831. doi: 10.1186/s13063-021-05752-1. Trials. 2021. PMID: 34814933 Free PMC article. Clinical Trial.
-
Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir.Fundam Clin Pharmacol. 2023 Aug;37(4):726-738. doi: 10.1111/fcp.12889. Epub 2023 Mar 25. Fundam Clin Pharmacol. 2023. PMID: 36931725 Free PMC article. Review.
-
Pharmacogenomics of Antiretroviral Drug Metabolism and Transport.Annu Rev Pharmacol Toxicol. 2021 Jan 6;61:565-585. doi: 10.1146/annurev-pharmtox-021320-111248. Epub 2020 Sep 22. Annu Rev Pharmacol Toxicol. 2021. PMID: 32960701 Free PMC article. Review.
References
-
- Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J. Acquir. Immune Defic. Syndr. 2006;41(2):194–200. - PubMed
-
- Jenh AM, Thio CL, Pham PA. Tenofovir for the treatment of hepatitis B virus. Pharmacotherapy. 2009;29(10):1212–1227. - PubMed
-
- Gallant JE, Dejesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N. Engl. J. Med. 2006;354(3):251–260. - PubMed
-
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J. Acquir. Immune Defic. Syndr. 2010;53(3):323–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
