MiR-6875-3p promotes the proliferation, invasion and metastasis of hepatocellular carcinoma via BTG2/FAK/Akt pathway
- PMID: 30621734
- PMCID: PMC6323674
- DOI: 10.1186/s13046-018-1020-z
MiR-6875-3p promotes the proliferation, invasion and metastasis of hepatocellular carcinoma via BTG2/FAK/Akt pathway
Abstract
Background: Increasing evidence supports the association of microRNA with tumor occurrence and development. However, the expression of miR-6875-3p and its role in cell proliferation, invasion and metastasis in hepatocellular carcinoma (HCC) remains elusive.
Methods: The expression of miR-6875-3p and BTG2 in HCC tissues and cell lines was detected by using in situ hybridization, immunohistochemistry and qRT-PCR, respectively. A western blot assay, qRT-PCR and Luciferase reporter assay were employed to study the interaction between miR-6875-3p and BTG2. Cell proliferation invasion and metastasis were measured by MTT, transwell and matrigel analyses in vitro. In vivo, tumorigenicity and metastasis assays were performed in nude mice.
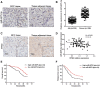
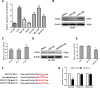
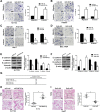
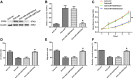
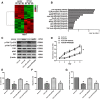
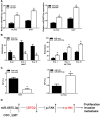
Results: We found that miR-6875-3p were elevated expressed in HCC tissues and cell lines, and negatively correlated with BTG2 expression, while positively correlated with tumor staging, size, degree of differentiation, and vascular invasion of HCC. Moreover, in vitro and in vivo assays showed that miR-6875-3p regulates EMT and improve the proliferation, metastasis and stem cell-like properties of HCC cells. BTG2 was identified as a direct and functional target of miR-6875-3p via the 3'-UTR of BTG2. We also confirmed that miR-6875-3p plays its biological functions via the BTG2/FAK/Akt pathway.
Conclusion: Our study provides evidence that high expression of miR-6875-3p can promote tumorigenesis of HCC in vitro and in vivo, so as to function as a novel oncogene in HCC. In mechanism, we found that miR-6875-3p plays its biological functions via the BTG2/FAK/Akt pathway.
Keywords: BTG2; HCC; Invasion; Metastasis; Proliferation; miR-6875-3p.
Conflict of interest statement
Ethics approval and consent to participate
Clinical data have been approved by the Ethics Committee of Second Affiliated Hospital of Jilin University and approved by the patients. All animal experiments were approved by Animal Care and Use Committee of Second Affiliated Hospital of Jilin University.
Consent for publication
All contributing authors agree to the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
MicroRNA-655-3p functions as a tumor suppressor by regulating ADAM10 and β-catenin pathway in Hepatocellular Carcinoma.J Exp Clin Cancer Res. 2016 Jun 4;35(1):89. doi: 10.1186/s13046-016-0368-1. J Exp Clin Cancer Res. 2016. PMID: 27259866 Free PMC article.
-
PRMT5 promotes cell proliferation by inhibiting BTG2 expression via the ERK signaling pathway in hepatocellular carcinoma.Cancer Med. 2018 Mar;7(3):869-882. doi: 10.1002/cam4.1360. Epub 2018 Feb 14. Cancer Med. 2018. PMID: 29441724 Free PMC article.
-
Methylation-associated silencing of microRNA-129-3p promotes epithelial-mesenchymal transition, invasion and metastasis of hepatocelluar cancer by targeting Aurora-A.Oncotarget. 2016 Nov 22;7(47):78009-78028. doi: 10.18632/oncotarget.12870. Oncotarget. 2016. PMID: 27793005 Free PMC article.
-
microRNA involvement in hepatocellular carcinoma.Anticancer Agents Med Chem. 2011 Jul;11(6):500-21. doi: 10.2174/187152011796011037. Anticancer Agents Med Chem. 2011. PMID: 21554203 Review.
-
The Epigenetic Regulation of HCC Metastasis.Int J Mol Sci. 2018 Dec 10;19(12):3978. doi: 10.3390/ijms19123978. Int J Mol Sci. 2018. PMID: 30544763 Free PMC article. Review.
Cited by
-
Circular RNA hsa_circ_101555 promotes hepatocellular carcinoma cell proliferation and migration by sponging miR-145-5p and regulating CDCA3 expression.Cell Death Dis. 2021 Apr 6;12(4):356. doi: 10.1038/s41419-021-03626-7. Cell Death Dis. 2021. PMID: 33824281 Free PMC article.
-
m6A-Induced LncRNA MEG3 Suppresses the Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cell Through miR-544b/BTG2 Signaling.Onco Targets Ther. 2021 Jun 15;14:3745-3755. doi: 10.2147/OTT.S289198. eCollection 2021. Onco Targets Ther. 2021. PMID: 34163177 Free PMC article.
-
miR-3677-5p promotes the proliferation, migration and invasion of hepatocellular carcinoma cells and is associated with prognosis.Exp Ther Med. 2021 Jul;22(1):780. doi: 10.3892/etm.2021.10212. Epub 2021 May 19. Exp Ther Med. 2021. PMID: 34055079 Free PMC article.
-
Hsa-miR-330-5p Aggravates Thyroid Carcinoma via Targeting FOXE1.J Oncol. 2021 Jul 2;2021:1070365. doi: 10.1155/2021/1070365. eCollection 2021. J Oncol. 2021. PMID: 34306074 Free PMC article.
-
Ribonucleic acid-binding protein CPSF6 promotes glycolysis and suppresses apoptosis in hepatocellular carcinoma cells by inhibiting the BTG2 expression.Biomed Eng Online. 2021 Jul 3;20(1):67. doi: 10.1186/s12938-021-00903-6. Biomed Eng Online. 2021. PMID: 34217312 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

