Poly-ADP-Ribosylation of Estrogen Receptor-Alpha by PARP1 Mediates Antiestrogen Resistance in Human Breast Cancer Cells
- PMID: 30621214
- PMCID: PMC6357000
- DOI: 10.3390/cancers11010043
Poly-ADP-Ribosylation of Estrogen Receptor-Alpha by PARP1 Mediates Antiestrogen Resistance in Human Breast Cancer Cells
Abstract
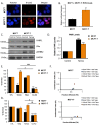
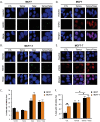
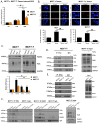
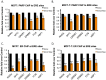
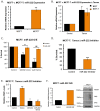
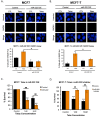
Therapeutic targeting of estrogen receptor-α (ERα) by the anti-estrogen tamoxifen is standard of care for premenopausal breast cancer patients and remains a key component of treatment strategies for postmenopausal patients. While tamoxifen significantly increases overall survival, tamoxifen resistance remains a major limitation despite continued expression of ERα in resistant tumors. Previous reports have described increased oxidative stress in tamoxifen resistant versus sensitive breast cancer and a role for PARP1 in mediating oxidative damage repair. We hypothesized that PARP1 activity mediated tamoxifen resistance in ERα-positive breast cancer and that combining the antiestrogen tamoxifen with a PARP1 inhibitor (PARPi) would sensitize tamoxifen resistant cells to tamoxifen therapy. In tamoxifen-resistant vs. -sensitive breast cancer cells, oxidative stress and PARP1 overexpression were increased. Furthermore, differential PARylation of ERα was observed in tamoxifen-resistant versus -sensitive cells, and ERα PARylation was increased by tamoxifen treatment. Loss of ERα PARylation following treatment with a PARP inhibitor (talazoparib) augmented tamoxifen sensitivity and decreased localization of both ERα and PARP1 to ERα-target genes. Co-administration of talazoparib plus tamoxifen increased DNA damage accumulation and decreased cell survival in a dose-dependent manner. The ability of PARPi to overcome tamoxifen resistance was dependent on ERα, as lack of ERα-mediated estrogen signaling expression and showed no response to tamoxifen-PARPi treatment. These results correlate ERα PARylation with tamoxifen resistance and indicate a novel mechanism-based approach to overcome tamoxifen resistance in ER+ breast cancer.
Keywords: PARP inhibitor; antiestrogen resistance; breast cancer; estrogen receptor; tamoxifen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance.Autophagy. 2009 Apr;5(3):400-3. doi: 10.4161/auto.5.3.7784. Epub 2009 Apr 7. Autophagy. 2009. PMID: 19221464
-
AKT3 regulates ErbB2, ErbB3 and estrogen receptor α expression and contributes to endocrine therapy resistance of ErbB2(+) breast tumor cells from Balb-neuT mice.Cell Signal. 2014 May;26(5):1021-9. doi: 10.1016/j.cellsig.2014.01.018. Epub 2014 Jan 24. Cell Signal. 2014. PMID: 24463007
-
Relationship between intratumoral expression of genes coding for xenobiotic-metabolizing enzymes and benefit from adjuvant tamoxifen in estrogen receptor alpha-positive postmenopausal breast carcinoma.Breast Cancer Res. 2004;6(3):R252-63. doi: 10.1186/bcr784. Epub 2004 Mar 26. Breast Cancer Res. 2004. PMID: 15084249 Free PMC article.
-
Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling.Oncogene. 2003 Oct 20;22(47):7316-39. doi: 10.1038/sj.onc.1206937. Oncogene. 2003. PMID: 14576841 Review.
-
From Micro to Long: Non-Coding RNAs in Tamoxifen Resistance of Breast Cancer Cells.Cancers (Basel). 2021 Jul 22;13(15):3688. doi: 10.3390/cancers13153688. Cancers (Basel). 2021. PMID: 34359587 Free PMC article. Review.
Cited by
-
Human PARP1 substrates and regulators of its catalytic activity: An updated overview.Front Pharmacol. 2023 Feb 23;14:1137151. doi: 10.3389/fphar.2023.1137151. eCollection 2023. Front Pharmacol. 2023. PMID: 36909172 Free PMC article. Review.
-
PARP1 as a Marker of an Aggressive Clinical Phenotype in Cutaneous Melanoma-A Clinical and an In Vitro Study.Cells. 2021 Jan 31;10(2):286. doi: 10.3390/cells10020286. Cells. 2021. PMID: 33572647 Free PMC article.
-
A novel group of genes that cause endocrine resistance in breast cancer identified by dynamic gene expression analysis.Oncotarget. 2022 Apr 6;13:600-613. doi: 10.18632/oncotarget.28225. eCollection 2022. Oncotarget. 2022. PMID: 35401937 Free PMC article.
-
Clinical Significance of PIK3CA and ESR1 Mutations in Circulating Tumor DNA: Analysis from the MONARCH 2 Study of Abemaciclib plus Fulvestrant.Clin Cancer Res. 2022 Apr 14;28(8):1500-1506. doi: 10.1158/1078-0432.CCR-21-3276. Clin Cancer Res. 2022. PMID: 35121623 Free PMC article. Clinical Trial.
-
Network-informed discovery of multidrug combinations for ERα+/HER2-/PI3Kα-mutant breast cancer.Cell Mol Life Sci. 2023 Mar 3;80(3):80. doi: 10.1007/s00018-023-04730-x. Cell Mol Life Sci. 2023. PMID: 36869202 Free PMC article.
References
-
- Jin K., Park S., Teo W.W., Korangath P., Cho S.S., Yoshida T., Gyorffy B., Goswami C.P., Nakshatri H., Cruz L.A., et al. HOXB7 Is an ERalpha Cofactor in the Activation of HER2 and Multiple ER Target Genes Leading to Endocrine Resistance. Cancer Discov. 2015;5:944–959. doi: 10.1158/2159-8290.CD-15-0090. - DOI - PMC - PubMed
-
- Bekele R.T., Venkatraman G., Liu R.Z., Tang X., Mi S., Benesch M.G., Mackey J.R., Godbout R., Curtis J.M., McMullen T.P., et al. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: Implications for tamoxifen therapy and resistance. Sci. Rep. 2016;6:21164. doi: 10.1038/srep21164. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

