KDELR2 Competes with Measles Virus Envelope Proteins for Cellular Chaperones Reducing Their Chaperone-Mediated Cell Surface Transport
- PMID: 30621148
- PMCID: PMC6356275
- DOI: 10.3390/v11010027
KDELR2 Competes with Measles Virus Envelope Proteins for Cellular Chaperones Reducing Their Chaperone-Mediated Cell Surface Transport
Abstract
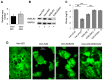
Recently, we found that the cytidine deaminase APOBEC3G (A3G) inhibits measles (MV) replication. Using a microarray, we identified differential regulation of several host genes upon ectopic expression of A3G. One of the up-regulated genes, the endoplasmic reticulum (ER) protein retention receptor KDELR2, reduced MV replication ~5 fold when it was over-expressed individually in Vero and CEM-SS T cells. Silencing of KDELR2 in A3G-expressing Vero cells abrogated the antiviral activity induced by A3G, confirming its role as an A3G-regulated antiviral host factor. Recognition of the KDEL (Lys-Asp-Glu-Leu) motif by KDEL receptors initiates the retrograde transport of soluble proteins that have escaped the ER and play an important role in ER quality control. Although KDELR2 over-expression reduced MV titers in cell cultures, we observed no interaction between KDELR2 and the MV hemagglutinin (H) protein. Instead, KDELR2 retained chaperones in the ER, which are required for the correct folding and transport of the MV envelope glycoproteins H and fusion protein (F) to the cell surface. Our data indicate that KDELR2 competes with MV envelope proteins for binding to calnexin and GRP78/Bip, and that this interaction limits the availability of the chaperones for MV proteins, causing the reduction of virus spread and titers.
Keywords: GRP78; KDELR2; calnexin; measles virus; surface transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
APOBEC3G-Regulated Host Factors Interfere with Measles Virus Replication: Role of REDD1 and Mammalian TORC1 Inhibition.J Virol. 2018 Aug 16;92(17):e00835-18. doi: 10.1128/JVI.00835-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925665 Free PMC article.
-
The measles virus (MV) glycoproteins interact with cellular chaperones in the endoplasmic reticulum and MV infection upregulates chaperone expression.Arch Virol. 2001;146(11):2055-68. doi: 10.1007/s007050170020. Arch Virol. 2001. PMID: 11765911
-
Cell-to-Cell Measles Virus Spread between Human Neurons Is Dependent on Hemagglutinin and Hyperfusogenic Fusion Protein.J Virol. 2018 Feb 26;92(6):e02166-17. doi: 10.1128/JVI.02166-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298883 Free PMC article.
-
Measles virus glycoprotein complex assembly, receptor attachment, and cell entry.Curr Top Microbiol Immunol. 2009;329:59-76. doi: 10.1007/978-3-540-70523-9_4. Curr Top Microbiol Immunol. 2009. PMID: 19198562 Free PMC article. Review.
-
Measles virus receptors.Curr Top Microbiol Immunol. 2009;329:13-30. doi: 10.1007/978-3-540-70523-9_2. Curr Top Microbiol Immunol. 2009. PMID: 19198560 Review.
Cited by
-
KDELR2 knockdown synergizes with temozolomide to induce glioma cell apoptosis through the CHOP and JNK/p38 pathways.Transl Cancer Res. 2021 Jul;10(7):3491-3506. doi: 10.21037/tcr-21-869. Transl Cancer Res. 2021. PMID: 35116653 Free PMC article.
-
KDEL Receptors: Pathophysiological Functions, Therapeutic Options, and Biotechnological Opportunities.Biomedicines. 2022 May 25;10(6):1234. doi: 10.3390/biomedicines10061234. Biomedicines. 2022. PMID: 35740256 Free PMC article. Review.
-
Morbilliviruses: Entry, Exit and Everything In-Between.Viruses. 2019 Nov 7;11(11):1036. doi: 10.3390/v11111036. Viruses. 2019. PMID: 31703308 Free PMC article.
References
-
- Fehrholz M., Kendl S., Prifert C., Weissbrich B., Lemon K., Rennick L., Duprex P.W., Rima B.K., Koning F.A., Holmes R.K., et al. The innate antiviral factor APOBEC3G targets replication of measles, mumps and respiratory syncytial viruses. Pt 3J. Gen. Virol. 2012;93:565–576. doi: 10.1099/vir.0.038919-0. - DOI - PubMed
-
- Pulvirenti T., Giannotta M., Capestrano M., Capitani M., Pisanu A., Polishchuk R.S., San Pietro E., Beznoussenko G.V., Mironov A.A., Turacchio G., et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 2008;10:912–922. doi: 10.1038/ncb1751. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

