Epigenetic and Transcriptional Regulation in the Induction, Maintenance, Heterogeneity, and Recall-Response of Effector and Memory Th2 Cells
- PMID: 30619290
- PMCID: PMC6299044
- DOI: 10.3389/fimmu.2018.02929
Epigenetic and Transcriptional Regulation in the Induction, Maintenance, Heterogeneity, and Recall-Response of Effector and Memory Th2 Cells
Abstract
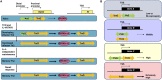
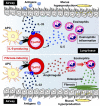
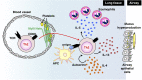
Antigen-primed T cells respond to restimulation much faster than naïve T cells and form the cellular basis of immunological memory. The formation of memory Th2 cells starts when naïve CD4 T cells are transformed into effector Th2 cells and is completed after antigen clearance and a long-term resting phase accompanied by epigenetic changes in the Th2 signature genes. Memory Th2 cells maintain their functions and acquired heterogeneity through epigenetic machinery, on which the recall-response of memory Th2 cells is also dependent. We provide an overview of the epigenetics in the whole Th2 cell cycle, mainly focusing on two different histone lysine methyltransferase complexes: the Polycomb and Trithorax groups. We finally discuss the pathophysiology and potential therapeutic strategies for the treatment of Th2-mediated inflammatory diseases in mice and humans.
Keywords: GATA3; airway infiammation; allergic disease; pathogenic Th2 (Tpath2) cells; polycomb and trithorax.
Figures

Similar articles
-
Th2 Cells in Health and Disease.Annu Rev Immunol. 2017 Apr 26;35:53-84. doi: 10.1146/annurev-immunol-051116-052350. Epub 2016 Nov 28. Annu Rev Immunol. 2017. PMID: 27912316 Review.
-
Initiation and maintenance of Th2 cell identity.Curr Opin Immunol. 2008 Jun;20(3):265-71. doi: 10.1016/j.coi.2008.03.011. Epub 2008 May 22. Curr Opin Immunol. 2008. PMID: 18502111 Review.
-
Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory.Semin Immunol. 2009 Apr;21(2):78-83. doi: 10.1016/j.smim.2009.02.001. Epub 2009 Mar 9. Semin Immunol. 2009. PMID: 19269851 Review.
-
CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm.Int Immunol. 2016 Apr;28(4):163-71. doi: 10.1093/intimm/dxw006. Epub 2016 Feb 12. Int Immunol. 2016. PMID: 26874355 Free PMC article. Review.
-
Menin Controls the Memory Th2 Cell Function by Maintaining the Epigenetic Integrity of Th2 Cells.J Immunol. 2017 Aug 1;199(3):1153-1162. doi: 10.4049/jimmunol.1602129. Epub 2017 Jun 28. J Immunol. 2017. PMID: 28659357
Cited by
-
Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease.J Exp Med. 2020 Sep 7;217(9):e20190865. doi: 10.1084/jem.20190865. J Exp Med. 2020. PMID: 32579670 Free PMC article.
-
Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis.Front Immunol. 2022 Aug 9;13:945063. doi: 10.3389/fimmu.2022.945063. eCollection 2022. Front Immunol. 2022. PMID: 36016937 Free PMC article. Review.
-
Understanding Asthma and Allergies by the Lens of Biodiversity and Epigenetic Changes.Front Immunol. 2021 Mar 1;12:623737. doi: 10.3389/fimmu.2021.623737. eCollection 2021. Front Immunol. 2021. PMID: 33732246 Free PMC article. Review.
-
Regulatory T-cells and IL-5 mediate pain outcomes in a preclinical model of chronic muscle pain.Mol Pain. 2023 Jan-Dec;19:17448069221110691. doi: 10.1177/17448069221110691. Mol Pain. 2023. PMID: 35712872 Free PMC article.
-
Epigenetic modulation of antitumor immunity for improved cancer immunotherapy.Mol Cancer. 2021 Dec 20;20(1):171. doi: 10.1186/s12943-021-01464-x. Mol Cancer. 2021. PMID: 34930302 Free PMC article. Review.
References
-
- Onodera A, Tumes DJ, Nakayama T. Epigenetic control of immune T cell memory. In: Bonifer C, Cockerill PN, editors. Transcriptional and Epigenetic Mechanisms Regulating Normal and Aberrant Blood Cell Development. Epigenetics and Human Health. (Berlin, Heidelberg: Springer; ). p. 367–82.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

