The Therapeutic Effect of STAT3 Signaling-Suppressed MSC on Pain and Articular Cartilage Damage in a Rat Model of Monosodium Iodoacetate-Induced Osteoarthritis
- PMID: 30619261
- PMCID: PMC6305125
- DOI: 10.3389/fimmu.2018.02881
The Therapeutic Effect of STAT3 Signaling-Suppressed MSC on Pain and Articular Cartilage Damage in a Rat Model of Monosodium Iodoacetate-Induced Osteoarthritis
Abstract
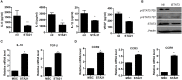
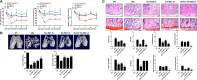
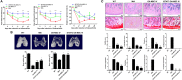
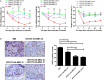
Osteoarthritis (OA) is a degenerative disease that induces pain, cartilage deformation, and joint inflammation. Mesenchymal stem cells (MSCs) are potential therapeutic agents for treatment of OA. However, MSC therapy can cause excessive inflammation. Signal transducer and activator of transcription 3 (STAT3) modulates secretion of many proinflammatory cytokines. Experimental OA was induced by intra-articular (IA) injection of monosodium iodoacetate (MIA) to the right knee of rats. MSCs from OA patients (OA-MSCs) were treated with STA21, a small molecule that blocks STAT3 signaling, by IA or intravenous (IV) injection after MIA injection. Pain severity was quantified by assessment of secondary tactile allodynia using the von Frey assessment test. Cartilage degradation was measured by microcomputed tomography image analysis, histological analysis, and the Mankin score. Protein and gene expression was evaluated by enzyme-linked immunosorbent assay, immunohistochemistry, and real-time polymerase chain reaction. MSCs increased production of proinflammatory cytokines under inflammatory conditions. STA21 significantly decreased expression of these proinflammatory molecules via inhibition of STAT3 activity but increased gene expression of molecules related to migration potential and immunomodulation in OA-MSCs. STAT3-inhibited OA-MSCs administrated by IV or IA injection decreased pain severity and cartilage damage in rats with MIA-induced OA rats by decreasing proinflammatory cytokines in the joints. Combined IA and IV-injected STAT3-inhibited OA-MSCs had an additive effect of pain relief in MIA-induced OA rats. STAT3 inhibition may optimize the therapeutic activities of MSCs for treating OA by attenuating pain and progression of MIA by inhibiting inflammation and cartilage damage.
Keywords: TRPV1; inflammation; mesenchymal stem cells; osteoarthritis; stat3.
Figures

Similar articles
-
Intraarticular injection of processed lipoaspirate cells has anti-inflammatory and analgesic effects but does not improve degenerative changes in murine monoiodoacetate-induced osteoarthritis.BMC Musculoskelet Disord. 2019 Jul 19;20(1):335. doi: 10.1186/s12891-019-2710-1. BMC Musculoskelet Disord. 2019. PMID: 31324245 Free PMC article.
-
Intraarticularly-Injected Mesenchymal Stem Cells Stimulate Anti-Inflammatory Molecules and Inhibit Pain Related Protein and Chondrolytic Enzymes in a Monoiodoacetate-Induced Rat Arthritis Model.Int J Mol Sci. 2018 Jan 9;19(1):203. doi: 10.3390/ijms19010203. Int J Mol Sci. 2018. PMID: 29315262 Free PMC article.
-
Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats.BMC Complement Altern Med. 2019 Nov 21;19(1):325. doi: 10.1186/s12906-019-2746-7. BMC Complement Altern Med. 2019. PMID: 31752825 Free PMC article.
-
Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage.Int J Mol Sci. 2023 Jun 9;24(12):9939. doi: 10.3390/ijms24129939. Int J Mol Sci. 2023. PMID: 37373096 Free PMC article. Review.
-
Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering.Acta Biomater. 2023 Sep 15;168:372-387. doi: 10.1016/j.actbio.2023.07.024. Epub 2023 Jul 21. Acta Biomater. 2023. PMID: 37481194 Review.
Cited by
-
Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing.Rev Endocr Metab Disord. 2024 Apr;25(2):339-367. doi: 10.1007/s11154-023-09860-y. Epub 2023 Dec 6. Rev Endocr Metab Disord. 2024. PMID: 38055160 Review.
-
Cell therapy for osteonecrosis of femoral head and joint preservation.J Clin Orthop Trauma. 2021 Nov 27;24:101713. doi: 10.1016/j.jcot.2021.101713. eCollection 2022 Jan. J Clin Orthop Trauma. 2021. PMID: 34926146 Free PMC article.
-
Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions.Bioact Mater. 2021 Nov 18;11:317-338. doi: 10.1016/j.bioactmat.2021.10.002. eCollection 2022 May. Bioact Mater. 2021. PMID: 34977434 Free PMC article. Review.
-
Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis.Stem Cell Res Ther. 2022 Jan 10;13(1):14. doi: 10.1186/s13287-021-02689-9. Stem Cell Res Ther. 2022. PMID: 35012666 Free PMC article. Review.
-
Endogenous CCN family member WISP1 inhibits trauma-induced heterotopic ossification.JCI Insight. 2020 Jul 9;5(13):e135432. doi: 10.1172/jci.insight.135432. JCI Insight. 2020. PMID: 32484792 Free PMC article.
References
-
- Kammermann JR, Kincaid SA, Rumph PF, Baird DK, Visco DM. Tumor necrosis factor-alpha (TNF-alpha) in canine osteoarthritis: immunolocalization of TNF-alpha, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthritis Cartilage (1996) 4:23–34. 10.1016/S1063-4584(96)80004-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

