Astrocyte-Mediated Neuromodulatory Regulation in Preclinical ALS: A Metadata Analysis
- PMID: 30618638
- PMCID: PMC6305074
- DOI: 10.3389/fncel.2018.00491
Astrocyte-Mediated Neuromodulatory Regulation in Preclinical ALS: A Metadata Analysis
Abstract
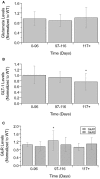
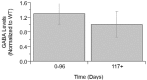
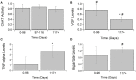
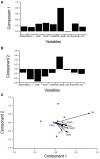
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease characterized by progressive degradation of motoneurons in the central nervous system (CNS). Astrocytes are key regulators for inflammation and neuromodulatory signaling, both of which contribute to ALS. The study goal was to ascertain potential temporal changes in astrocyte-mediated neuromodulatory regulation with transgenic ALS model progression: glutamate, GTL-1, GluR1, GluR2, GABA, ChAT activity, VGF, TNFα, aspartate, and IGF-1. We examine neuromodulatory changes in data aggregates from 42 peer-reviewed studies derived from transgenic ALS mixed cell cultures (neurons + astrocytes). For each corresponding experimental time point, the ratio of transgenic to wild type (WT) was found for each compound. ANOVA and a student's t-test were performed to compare disease stages (early, post-onset, and end stage). Glutamate in transgenic SOD1-G93A mixed cell cultures does not change over time (p > 0.05). GLT-1 levels were found to be decreased 23% over WT but only at end-stage (p < 0.05). Glutamate receptors (GluR1, GluR2) in SOD1-G93A were not substantially different from WT, although SOD1-G93A GluR1 decreased by 21% from post-onset to end-stage (p < 0.05). ChAT activity was insignificantly decreased. VGF is decreased throughout ALS (p < 0.05). Aspartate is elevated by 25% in SOD1-G93A but only during end-stage (p < 0.05). TNFα is increased by a dramatic 362% (p < 0.05). Furthermore, principal component analysis identified TNFα as contributing to 55% of the data variance in the first component. Thus, TNFα, which modulates astrocyte regulation via multiple pathways, could be a strategic treatment target. Overall results suggest changes in neuromodulator levels are subtle in SOD1-G93A ALS mixed cell cultures. If excitotoxicity is present as is often presumed, it could be due to ALS cells being more sensitive to small changes in neuromodulation. Hence, seemingly unsubstantial or oscillatory changes in neuromodulators could wreak havoc in ALS cells, resulting in failed microenvironment homeostasis whereby both hyperexcitability and hypoexcitability can coexist. Future work is needed to examine local, spatiotemporal neuromodulatory homeostasis and assess its functional impact in ALS.
Keywords: ChAT; GABA; GLT-1; TNFα; VGF; aspartate; glutamate.
Figures


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Undernutrition as a risk factor for tuberculosis disease.Cochrane Database Syst Rev. 2024 Jun 11;6(6):CD015890. doi: 10.1002/14651858.CD015890.pub2. Cochrane Database Syst Rev. 2024. PMID: 38860538 Free PMC article. Review.
Cited by
-
Involvement of Astrocytes and microRNA Dysregulation in Neurodegenerative Diseases: From Pathogenesis to Therapeutic Potential.Front Mol Neurosci. 2021 Mar 17;14:556215. doi: 10.3389/fnmol.2021.556215. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33815055 Free PMC article.
-
Research Progress on the Effects of Different Exercise Modes on the Secretion of Exerkines After Spinal Cord Injury.Cell Mol Neurobiol. 2024 Oct 1;44(1):62. doi: 10.1007/s10571-024-01497-y. Cell Mol Neurobiol. 2024. PMID: 39352588 Free PMC article. Review.
-
Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders.Cells. 2022 Mar 28;11(7):1139. doi: 10.3390/cells11071139. Cells. 2022. PMID: 35406702 Free PMC article. Review.
-
Molecular Chaperones' Potential against Defective Proteostasis of Amyotrophic Lateral Sclerosis.Cells. 2023 May 2;12(9):1302. doi: 10.3390/cells12091302. Cells. 2023. PMID: 37174703 Free PMC article. Review.
-
Mechanisms of TDP-43 Proteinopathy Onset and Propagation.Int J Mol Sci. 2021 Jun 2;22(11):6004. doi: 10.3390/ijms22116004. Int J Mol Sci. 2021. PMID: 34199367 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

