Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses
- PMID: 30618085
- PMCID: PMC6431606
- DOI: 10.1002/sctm.18-0038
Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses
Abstract
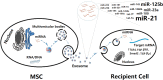
Radiation-induced lung injury (RILI) is a common complication in radiotherapy of thoracic tumors and limits the therapeutic dose of radiation that can be given to effectively control tumors. RILI develops through a complex pathological process, resulting in induction and activation of various cytokines, infiltration by inflammatory cells, cytokine-induced activation of fibroblasts, and subsequent tissue remodeling by activated fibroblasts, ultimately leading to impaired lung function and respiratory failure. Increasing evidence shows that mesenchymal stem cells (MSCs) may play a main role in modulating inflammation and immune responses, promoting survival and repair of damaged resident cells and enhancing regeneration of damaged tissue through soluble paracrine factors and therapeutic extracellular vesicles. Therefore, the use of the MSC-derived secretome and exosomes holds promising potential for RILI therapy. Here, we review recent progress on the potential mechanisms of MSC therapy for RILI, with an emphasis on soluble paracrine factors of MSCs. Hypotheses on how MSC derived exosomes or MSC-released exosomal miRNAs could attenuate RILI are also proposed. Problems and translational challenges of the therapies based on the MSC-derived secretome and exosomes are further summarized and underline the need for caution on rapid clinical translation. Stem Cells Translational Medicine 2019;8:344-354.
Keywords: Exosome; Lung fibrosis; Mesenchymal stem cells; Radiation pneumonitis; Secretome.
© 2019 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures

Similar articles
-
Mesenchymal stem cell-based therapy for radiation-induced lung injury.Stem Cell Res Ther. 2018 Jan 31;9(1):18. doi: 10.1186/s13287-018-0776-6. Stem Cell Res Ther. 2018. PMID: 29386045 Free PMC article. Review.
-
Applications and therapeutic mechanisms of action of mesenchymal stem cells in radiation-induced lung injury.Stem Cell Res Ther. 2021 Mar 25;12(1):212. doi: 10.1186/s13287-021-02279-9. Stem Cell Res Ther. 2021. PMID: 33766127 Free PMC article. Review.
-
Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability.Cell Physiol Biochem. 2017;44(4):1295-1310. doi: 10.1159/000485490. Epub 2017 Nov 29. Cell Physiol Biochem. 2017. PMID: 29183009
-
Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats.Cytotherapy. 2015 May;17(5):560-70. doi: 10.1016/j.jcyt.2015.02.011. Epub 2015 Mar 17. Cytotherapy. 2015. PMID: 25791071
-
The secretome of mesenchymal stem cells and oxidative stress: challenges and opportunities in cell-free regenerative medicine.Mol Biol Rep. 2021 Jul;48(7):5607-5619. doi: 10.1007/s11033-021-06360-7. Epub 2021 Jun 30. Mol Biol Rep. 2021. PMID: 34191238 Review.
Cited by
-
Rapid and effective preparation of clonal bone marrow-derived mesenchymal stem/stromal cell sheets to reduce renal fibrosis.Sci Rep. 2023 Mar 17;13(1):4421. doi: 10.1038/s41598-023-31437-7. Sci Rep. 2023. PMID: 36932137 Free PMC article.
-
Intratracheal Transplantation of Amnion-Derived Mesenchymal Stem Cells Ameliorates Hyperoxia-Induced Neonatal Hyperoxic Lung Injury via Aminoacyl-Peptide Hydrolase.Int J Stem Cells. 2020 Jul 30;13(2):221-236. doi: 10.15283/ijsc19110. Int J Stem Cells. 2020. PMID: 32323511 Free PMC article.
-
Mesenchymal Stem Cells for Mitigating Radiotherapy Side Effects.Cells. 2021 Feb 1;10(2):294. doi: 10.3390/cells10020294. Cells. 2021. PMID: 33535574 Free PMC article. Review.
-
Mesenchymal Stem Cells in Radiation-Induced Pulmonary Fibrosis: Future Prospects.Cells. 2022 Dec 20;12(1):6. doi: 10.3390/cells12010006. Cells. 2022. PMID: 36611801 Free PMC article. Review.
-
Therapeutic implications of exosomes in the treatment of radiation injury.Burns Trauma. 2022 Jan 21;10:tkab043. doi: 10.1093/burnst/tkab043. eCollection 2022. Burns Trauma. 2022. PMID: 35071650 Free PMC article. Review.
References
-
- Jeremic B, Fidarova E, Sharma V et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol 2015;116:21–26. - PubMed
-
- Graves PR, Siddiqui F, Anscher MS et al. Radiation pulmonary toxicity: From mechanisms to management. Semin Radiat Oncol 2010;20:201–207. - PubMed
-
- Marks LB, Yu X, Vujaskovic Z et al. Radiation‐induced lung injury. Semin Radiat Oncol 2003;13:333–345. - PubMed
-
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non‐small‐cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

