Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor
- PMID: 30611736
- PMCID: PMC9206443
- DOI: 10.1016/j.exer.2019.01.001
Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor
Abstract
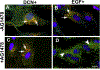
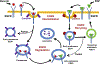
Decorin (Dcn), a small leucine-rich proteoglycan, is involved in the regulation of corneal wound healing. Epidermal growth factor receptor (EGFR) plays a critical role in corneal fibroblasts proliferation, migration and extracellular matrix (ECM) modulation upon injury or infection. The present study aimed to investigate the mechanistic role of Dcn in EGFR internalization to the regulation of corneal stromal fibroblasts (CSFs) migration, a key step in the corneal wound healing. Human corneal stromal fibroblasts (hCSF) cultures were generated from donor corneas. At 70% confluence, cells were switched to serum-free conditions for 48 h and then treated with decorin (250 nM) in the presence or absence of EGF (100 ng/ml) for various time points (10-60 min). Cell lysates were subjected to proteome array analysis screening for 42 different phosphorylated human receptor tyrosine kinases (RTKs), immunocytochemistry, and western blots to analyze EGFR phosphorylation. The scratch-wound assay was performed to evaluate the effects of decorin on EGF-mediated hCSF migration. Dcn caused a rapid EGFR phosphorylation within 10 min of exposure in RTK blot defining its role as a biological ligand for EGFR in hCSFs. Prolonged exposure to Dcn caused complete disappearance of EGFR and inhibition of the hCSF migration in the scratch wound assay suggesting Dcn binding to EGFR causes EGFR down-regulation. Immunostaining studies indicated that Dcn-treatment to hCSFs internalizes Dcn-EGFR complex, which does not require tyrosine kinase activity when treated with the AG1478 inhibitor and co-localizes the complex to the perinuclear region. Next, we found that Dcn-EGFR complex does not follow canonical early endosome internalization as revealed by the EEA1 antibody instead binds to the CD63 antibody directed for degradation by the late endosome. We also found that Dcn regulates the EGFR recycling by preventing its binding to Rab11, a specific antibody for recycling endosome. Further, hCSFs-pretreated with pharmacological inhibitors, methyl-β-cyclodextrin and chlorpromazine and supplemented with Dcn suggested EGFR trafficking via the caveolae-mediated pathway. These results suggest that Dcn acts as a biological ligand for EGFR and modulates hCSF migration via EGFR down-regulation, thus playing a vital role in corneal wound healing.
Keywords: Caveolae; Corneal wound healing; Decorin; Endocytosis; Epidermal growth factor receptor.
Copyright © 2019. Published by Elsevier Ltd.
Figures
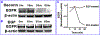

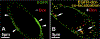




Similar articles
-
Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors.Endocrinology. 2008 Dec;149(12):6187-97. doi: 10.1210/en.2008-0780. Epub 2008 Aug 14. Endocrinology. 2008. PMID: 18703624
-
Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo.Exp Eye Res. 2022 Mar;216:108933. doi: 10.1016/j.exer.2022.108933. Epub 2022 Jan 11. Exp Eye Res. 2022. PMID: 35031282 Free PMC article.
-
Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells.Invest Ophthalmol Vis Sci. 2004 Mar;45(3):813-20. doi: 10.1167/iovs.03-0851. Invest Ophthalmol Vis Sci. 2004. PMID: 14985295 Free PMC article.
-
Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: The role of decorin.Cell Adh Migr. 2016 Mar 3;10(1-2):111-25. doi: 10.1080/19336918.2015.1106669. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26745663 Free PMC article. Review.
-
Restraint of Trophoblast Invasion of the Uterus by Decorin: Role in Pre-eclampsia.Am J Reprod Immunol. 2016 Mar;75(3):351-60. doi: 10.1111/aji.12449. Epub 2015 Nov 11. Am J Reprod Immunol. 2016. PMID: 26554635 Review.
Cited by
-
Gelam honey promotes ex vivo corneal fibroblasts wound healing.Cytotechnology. 2019 Dec;71(6):1121-1135. doi: 10.1007/s10616-019-00349-8. Epub 2019 Oct 12. Cytotechnology. 2019. PMID: 31606844 Free PMC article.
-
Adding exogenous biglycan or decorin improves tendon formation for equine peritenon and tendon proper cells in vitro.BMC Musculoskelet Disord. 2020 Sep 23;21(1):627. doi: 10.1186/s12891-020-03650-2. BMC Musculoskelet Disord. 2020. PMID: 32967653 Free PMC article.
-
Small leucine rich proteoglycans: Biology, function and their therapeutic potential in the ocular surface.Ocul Surf. 2023 Jul;29:521-536. doi: 10.1016/j.jtos.2023.06.013. Epub 2023 Jun 22. Ocul Surf. 2023. PMID: 37355022 Free PMC article. Review.
-
Novel insights into gene therapy in the cornea.Exp Eye Res. 2021 Jan;202:108361. doi: 10.1016/j.exer.2020.108361. Epub 2020 Nov 16. Exp Eye Res. 2021. PMID: 33212142 Free PMC article. Review.
-
A rabbit model for evaluating ocular damage from acrolein toxicity in vivo.Ann N Y Acad Sci. 2020 Nov;1480(1):233-245. doi: 10.1111/nyas.14514. Epub 2020 Oct 16. Ann N Y Acad Sci. 2020. PMID: 33067838 Free PMC article.
References
-
- Bakker J, Spits M, Neefjes J, Berlin I, 2017. The EGFR odyssey-from activation to destruction in space and time. J. Cell Sci. 130, 4087–4096. - PubMed
-
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E, 1992. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360, 361–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

