Exploring Novel Functions of the Small GTPase Ypt1p under Heat-Shock by Characterizing a Temperature-Sensitive Mutant Yeast Strain, ypt1-G80D
- PMID: 30609659
- PMCID: PMC6337079
- DOI: 10.3390/ijms20010132
Exploring Novel Functions of the Small GTPase Ypt1p under Heat-Shock by Characterizing a Temperature-Sensitive Mutant Yeast Strain, ypt1-G80D
Abstract
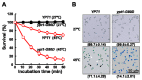
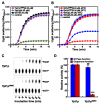
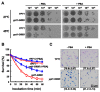
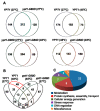
In our previous study, we found that Ypt1p, a Rab family small GTPase protein, exhibits a stress-driven structural and functional switch from a GTPase to a molecular chaperone, and mediates thermo tolerance in Saccharomyces cerevisiae. In the current study, we focused on the temperature-sensitive ypt1-G80D mutant, and found that the mutant cells are highly sensitive to heat-shock, due to a deficiency in the chaperone function of Ypt1pG80D. This defect results from an inability of the protein to form high molecular weight polymers, even though it retains almost normal GTPase function. The heat-stress sensitivity of ypt1-G80D cells was partially recovered by treatment with 4-phenylbutyric acid, a chemical chaperone. These findings indicate that loss of the chaperone function of Ypt1pG80D underlies the heat sensitivity of ypt1-G80D cells. We also compared the proteomes of YPT1 (wild-type) and ypt1-G80D cells to investigate Ypt1p-controlled proteins under heat-stress conditions. Our findings suggest that Ypt1p controls an abundance of proteins involved in metabolism, protein synthesis, cellular energy generation, stress response, and DNA regulation. Finally, we suggest that Ypt1p essentially regulates fundamental cellular processes under heat-stress conditions by acting as a molecular chaperone.
Keywords: functional switch; heat-shock; molecular chaperone; small GTPase; structural change.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Stress-driven structural and functional switching of Ypt1p from a GTPase to a molecular chaperone mediates thermo tolerance in Saccharomyces cerevisiae.FASEB J. 2015 Nov;29(11):4424-34. doi: 10.1096/fj.15-270140. Epub 2015 Jul 13. FASEB J. 2015. PMID: 26169936
-
Mutational analysis of the putative effector domain of the GTP-binding Ypt1 protein in yeast suggests specific regulation by a novel GAP activity.EMBO J. 1991 Apr;10(4):785-92. doi: 10.1002/j.1460-2075.1991.tb08010.x. EMBO J. 1991. PMID: 2009858 Free PMC article.
-
Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs.J Biol Chem. 2002 Oct 25;277(43):41023-31. doi: 10.1074/jbc.M205783200. Epub 2002 Aug 19. J Biol Chem. 2002. PMID: 12189143
-
Yeast rab GTPase-activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p.Mol Biol Cell. 2001 May;12(5):1215-26. doi: 10.1091/mbc.12.5.1215. Mol Biol Cell. 2001. PMID: 11359917 Free PMC article.
-
GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport.Mol Cell Biol. 1998 Feb;18(2):827-38. doi: 10.1128/MCB.18.2.827. Mol Cell Biol. 1998. PMID: 9447979 Free PMC article.
Cited by
-
Characterization of AtBAG2 as a Novel Molecular Chaperone.Life (Basel). 2023 Mar 3;13(3):687. doi: 10.3390/life13030687. Life (Basel). 2023. PMID: 36983842 Free PMC article.
-
Diverse Physiological Functions and Regulatory Mechanisms for Signal-Transducing Small GTPases.Int J Mol Sci. 2020 Oct 2;21(19):7291. doi: 10.3390/ijms21197291. Int J Mol Sci. 2020. PMID: 33023216 Free PMC article.
-
Focus on the Small GTPase Rab1: A Key Player in the Pathogenesis of Parkinson's Disease.Int J Mol Sci. 2021 Nov 8;22(21):12087. doi: 10.3390/ijms222112087. Int J Mol Sci. 2021. PMID: 34769517 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

