Cardiac Fibrotic Remodeling on a Chip with Dynamic Mechanical Stimulation
- PMID: 30609312
- PMCID: PMC6546425
- DOI: 10.1002/adhm.201801146
Cardiac Fibrotic Remodeling on a Chip with Dynamic Mechanical Stimulation
Abstract
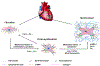
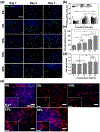
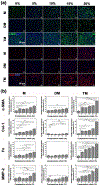
Cardiac tissue is characterized by being dynamic and contractile, imparting the important role of biomechanical cues in the regulation of normal physiological activity or pathological remodeling. However, the dynamic mechanical tension ability also varies due to extracellular matrix remodeling in fibrosis, accompanied with the phenotypic transition from cardiac fibroblasts (CFs) to myofibroblasts. It is hypothesized that the dynamic mechanical tension ability regulates cardiac phenotypic transition within fibrosis in a strain-mediated manner. In this study, a microdevice that is able to simultaneously and accurately mimic the biomechanical properties of the cardiac physiological and pathological microenvironment is developed. The microdevice can apply cyclic compressions with gradient magnitudes (5-20%) and tunable frequency onto gelatin methacryloyl (GelMA) hydrogels laden with CFs, and also enables the integration of cytokines. The strain-response correlations between mechanical compression and CFs spreading, and proliferation and fibrotic phenotype remolding, are investigated. Results reveal that mechanical compression plays a crucial role in the CFs phenotypic transition, depending on the strain of mechanical load and myofibroblast maturity of CFs encapsulated in GelMA hydrogels. The results provide evidence regarding the strain-response correlation of mechanical stimulation in CFs phenotypic remodeling, which can be used to develop new preventive or therapeutic strategies for cardiac fibrosis.
Keywords: cardiac fibrosis; hydrogels; mechanical stimulation; organ-on-a-chip; transforming growth factor-β.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


Similar articles
-
Mechanical stress regulates the mechanotransduction and metabolism of cardiac fibroblasts in fibrotic cardiac diseases.Eur J Cell Biol. 2023 Jun;102(2):151288. doi: 10.1016/j.ejcb.2023.151288. Epub 2023 Jan 18. Eur J Cell Biol. 2023. PMID: 36696810 Review.
-
Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling.Adv Healthc Mater. 2017 Jun;6(11):10.1002/adhm.201601434. doi: 10.1002/adhm.201601434. Epub 2017 May 12. Adv Healthc Mater. 2017. PMID: 28498548 Free PMC article.
-
A three-dimensional in vitro dynamic micro-tissue model of cardiac scar formation.Integr Biol (Camb). 2018 Mar 1;10(3):174-183. doi: 10.1039/c7ib00199a. Epub 2018 Mar 13. Integr Biol (Camb). 2018. PMID: 29532839
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies.Adv Drug Deliv Rev. 2021 Jun;173:504-519. doi: 10.1016/j.addr.2021.03.021. Epub 2021 Apr 5. Adv Drug Deliv Rev. 2021. PMID: 33831476 Free PMC article. Review.
Cited by
-
In Vitro Mechanical Stimulation to Reproduce the Pathological Hallmarks of Human Cardiac Fibrosis on a Beating Chip and Predict The Efficacy of Drugs and Advanced Therapies.Adv Healthc Mater. 2024 Feb;13(4):e2301481. doi: 10.1002/adhm.202301481. Epub 2023 Nov 27. Adv Healthc Mater. 2024. PMID: 37941521 Free PMC article.
-
Current strategies of mechanical stimulation for maturation of cardiac microtissues.Biophys Rev. 2021 Sep 10;13(5):717-727. doi: 10.1007/s12551-021-00841-6. eCollection 2021 Oct. Biophys Rev. 2021. PMID: 34765047 Free PMC article. Review.
-
Organ-on-a-chip technology: a novel approach to investigate cardiovascular diseases.Cardiovasc Res. 2021 Dec 17;117(14):2742-2754. doi: 10.1093/cvr/cvab088. Cardiovasc Res. 2021. PMID: 33729461 Free PMC article. Review.
-
Mechanical Cues Regulating Proangiogenic Potential of Human Mesenchymal Stem Cells through YAP-Mediated Mechanosensing.Small. 2020 Jun;16(25):e2001837. doi: 10.1002/smll.202001837. Epub 2020 May 17. Small. 2020. PMID: 32419312 Free PMC article.
-
Disease-inspired tissue engineering: Investigation of cardiovascular pathologies.ACS Biomater Sci Eng. 2020 May 11;6(5):2518-2532. doi: 10.1021/acsbiomaterials.9b01067. Epub 2019 Oct 29. ACS Biomater Sci Eng. 2020. PMID: 32974421 Free PMC article. Review.
References
-
- Porter KE, Turner NA, Pharmacology & therapeutics 2009, 123, 255; C. A. Souders, S. L. Bowers, T. A. Baudino, Circ Res 2009, 105, 1164. - PubMed
-
- Yokoyama T, Sekiguchi K, Tanaka T, Tomaru K, Arai M, Suzuki T, Nagai R, Journal of Cardiac Failure 1999, 5, 1968; A. Leask, Cardiovascular research 2007, 74, 207; R. Mazhari, J. H. Omens, J. W. Covell, A. D. McCulloch, Cardiovascular research 2000, 47, 284. - PubMed
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, Nature reviews. Molecular cell biology 2002, 3, 349. - PubMed
-
- Hinz B, The Journal of investigative dermatology 2007, 127, 526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

