Structures of TRPV2 in distinct conformations provide insight into role of the pore turret
- PMID: 30598551
- PMCID: PMC6458597
- DOI: 10.1038/s41594-018-0168-8
Structures of TRPV2 in distinct conformations provide insight into role of the pore turret
Abstract
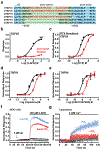
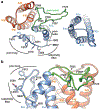
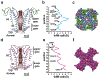
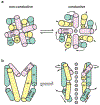
Cation channels of the transient receptor potential (TRP) family serve important physiological roles by opening in response to diverse intra- and extracellular stimuli that regulate their lower or upper gates. Despite extensive studies, the mechanism coupling these gates has remained obscure. Previous structures have failed to resolve extracellular loops, known in the TRPV subfamily as 'pore turrets', which are proximal to the upper gates. We established the importance of the pore turret through activity assays and by solving structures of rat TRPV2, both with and without an intact turret at resolutions of 4.0 Å and 3.6 Å, respectively. These structures resolve the full-length pore turret and reveal fully open and partially open states of TRPV2, both with unoccupied vanilloid pockets. Our results suggest a mechanism by which physiological signals, such as lipid binding, can regulate the lower gate and couple to the upper gate through a pore-turret-facilitated mechanism.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

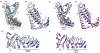
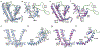
Similar articles
-
Structure of the full-length TRPV2 channel by cryo-EM.Nat Commun. 2016 Mar 29;7:11130. doi: 10.1038/ncomms11130. Nat Commun. 2016. PMID: 27021073 Free PMC article.
-
TRPV1 structures in distinct conformations reveal activation mechanisms.Nature. 2013 Dec 5;504(7478):113-8. doi: 10.1038/nature12823. Nature. 2013. PMID: 24305161 Free PMC article.
-
The regulatory mechanism of mammalian TRPMLs revealed by cryo-EM.FEBS J. 2018 Jul;285(14):2579-2585. doi: 10.1111/febs.14443. Epub 2018 Apr 14. FEBS J. 2018. PMID: 29577631 Free PMC article. Review.
-
Identification of a helix-turn-helix motif for high temperature dependence of vanilloid receptor TRPV2.J Physiol. 2021 Nov;599(21):4831-4844. doi: 10.1113/JP282073. Epub 2021 Oct 3. J Physiol. 2021. PMID: 34605028
-
Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli.J Endocrinol. 2006 Dec;191(3):515-23. doi: 10.1677/joe.1.07000. J Endocrinol. 2006. PMID: 17170210 Review.
Cited by
-
Thermal gradient ring for analysis of temperature-dependent behaviors involving TRP channels in mice.J Physiol Sci. 2024 Feb 8;74(1):9. doi: 10.1186/s12576-024-00903-w. J Physiol Sci. 2024. PMID: 38331738 Free PMC article. Review.
-
Structure-Function Relationship and Physiological Roles of Transient Receptor Potential Canonical (TRPC) 4 and 5 Channels.Cells. 2019 Dec 27;9(1):73. doi: 10.3390/cells9010073. Cells. 2019. PMID: 31892199 Free PMC article. Review.
-
Structural insights into TRPV2 activation by small molecules.Nat Commun. 2022 Apr 28;13(1):2334. doi: 10.1038/s41467-022-30083-3. Nat Commun. 2022. PMID: 35484159 Free PMC article.
-
Allosteric Antagonist Modulation of TRPV2 by Piperlongumine Impairs Glioblastoma Progression.ACS Cent Sci. 2021 May 26;7(5):868-881. doi: 10.1021/acscentsci.1c00070. Epub 2021 Apr 14. ACS Cent Sci. 2021. PMID: 34079902 Free PMC article.
-
Role of mechanically-sensitive cation channels Piezo1 and TRPV4 in trabecular meshwork cell mechanotransduction.Hum Cell. 2024 Mar;37(2):394-407. doi: 10.1007/s13577-024-01035-4. Epub 2024 Feb 5. Hum Cell. 2024. PMID: 38316716 Review.
References
-
- Clapham DE TRP channels as cellular sensors. Nature 426, 517–24 (2003). - PubMed
-
- Clapham DE, Runnels LW & Strubing C The TRP ion channel family. Nat Rev Neurosci 2, 387–96 (2001). - PubMed
-
- Montell C et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 9, 229–31 (2002). - PubMed
-
- Jordt SE & Ehrlich BE TRP channels in disease. Subcell Biochem 45, 253–71 (2007). - PubMed
-
- Nilius B TRP channels in disease. Biochim Biophys Acta 1772, 805–12 (2007). - PubMed
Methods-only references
-
- Mindell JA & Grigorieff N Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 142, 334–47 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

