Circulating blood cells and extracellular vesicles in acute cardioprotection
- PMID: 30590395
- PMCID: PMC6529916
- DOI: 10.1093/cvr/cvy314
Circulating blood cells and extracellular vesicles in acute cardioprotection
Abstract
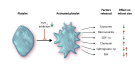
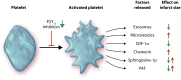
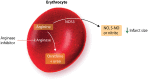
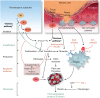
During an ST-elevation myocardial infarction (STEMI), the myocardium undergoes a prolonged period of ischaemia. Reperfusion therapy is essential to minimize cardiac injury but can paradoxically cause further damage. Experimental procedures to limit ischaemia and reperfusion (IR) injury have tended to focus on the cardiomyocytes since they are crucial for cardiac function. However, there is increasing evidence that non-cardiomyocyte resident cells in the heart (as discussed in a separate review in this Spotlight series) as well as circulating cells and factors play important roles in this pathology. For example, erythrocytes, in addition to their main oxygen-ferrying role, can protect the heart from IR injury via the export of nitric oxide bioactivity. Platelets are well-known to be involved in haemostasis and thrombosis, but beyond these roles, they secrete numerous factors including sphingosine-1 phosphate (S1P), platelet activating factor, and cytokines that can all strongly influence the development of IR injury. This is particularly relevant given that most STEMI patients receive at least one type of platelet inhibitor. Moreover, there are large numbers of circulating vesicles in the blood, including microvesicles and exosomes, which can exert both beneficial and detrimental effects on IR injury. Some of these effects are mediated by the transfer of microRNA (miRNA) to the heart. Synthetic miRNA molecules may offer an alternative approach to limiting the response to IR injury. We discuss these and other circulating factors, focussing on potential therapeutic targets relevant to IR injury. Given the prevalence of comorbidities such as diabetes in the target patient population, their influence will also be discussed. This article is part of a Cardiovascular Research Spotlight Issue entitled 'Cardioprotection Beyond the Cardiomyocyte', and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
Keywords: Cardioprotection; Exosomes; Haematopoietic cells; Ischaemia; Reperfusion.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2018. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Innate immunity as a target for acute cardioprotection.Cardiovasc Res. 2019 Jun 1;115(7):1131-1142. doi: 10.1093/cvr/cvy304. Cardiovasc Res. 2019. PMID: 30576455 Free PMC article. Review.
-
The coronary circulation in acute myocardial ischaemia/reperfusion injury: a target for cardioprotection.Cardiovasc Res. 2019 Jun 1;115(7):1143-1155. doi: 10.1093/cvr/cvy286. Cardiovasc Res. 2019. PMID: 30428011 Free PMC article. Review.
-
Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection.Cardiovasc Res. 2019 Jun 1;115(7):1167-1177. doi: 10.1093/cvr/cvz053. Cardiovasc Res. 2019. PMID: 30796814 Free PMC article. Review.
-
Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target.Cardiovasc Res. 2019 Jun 1;115(7):1178-1188. doi: 10.1093/cvr/cvz070. Cardiovasc Res. 2019. PMID: 30906948 Free PMC article. Review.
-
Translation of experimental cardioprotective capability of P2Y12 inhibitors into clinical outcome in patients with ST-elevation myocardial infarction.Basic Res Cardiol. 2021 May 26;116(1):36. doi: 10.1007/s00395-021-00870-y. Basic Res Cardiol. 2021. PMID: 34037861
Cited by
-
Atypical Roles of the Chemokine Receptor ACKR3/CXCR7 in Platelet Pathophysiology.Cells. 2022 Jan 9;11(2):213. doi: 10.3390/cells11020213. Cells. 2022. PMID: 35053329 Free PMC article. Review.
-
COVID-19 and erythrocrine function: The roller coaster and danger.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221103151. doi: 10.1177/03946320221103151. Int J Immunopathol Pharmacol. 2022. PMID: 35590466 Free PMC article.
-
Evaluating Novel Targets of Ischemia Reperfusion Injury in Pig Models.Int J Mol Sci. 2019 Sep 25;20(19):4749. doi: 10.3390/ijms20194749. Int J Mol Sci. 2019. PMID: 31557793 Free PMC article. Review.
-
Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles.Cardiovasc Res. 2023 Mar 17;119(1):45-63. doi: 10.1093/cvr/cvac031. Cardiovasc Res. 2023. PMID: 35325061 Free PMC article.
-
Sex is no determinant of cardioprotection by ischemic preconditioning in rats, but ischemic/reperfused tissue mass is for remote ischemic preconditioning.Physiol Rep. 2019 Jul;7(12):e14146. doi: 10.14814/phy2.14146. Physiol Rep. 2019. PMID: 31210033 Free PMC article.
References
-
- Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. - PubMed
-
- Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D.. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 2016;111:70.. - PMC - PubMed
-
- Perrino C, Barabasi AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, Lecour S, Leor J, Madonna R, Mayr M, Prunier F, Sluijter JPG, Schulz R, Thum T, Ytrehus K, Ferdinandy P.. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:725–736. - PMC - PubMed
-
- Varga ZV, Giricz Z, Bencsik P, Madonna R, Gyongyosi M, Schulz R, Mayr M, Thum T, Puskas LG, Ferdinandy P.. Functional genomics of cardioprotection by ischemic conditioning and the influence of comorbid conditions: implications in target identification. Curr Drug Targets 2015;16:904–911. - PubMed

