Cowpea chlorotic mottle bromovirus replication proteins support template-selective RNA replication in Saccharomyces cerevisiae
- PMID: 30586378
- PMCID: PMC6306254
- DOI: 10.1371/journal.pone.0208743
Cowpea chlorotic mottle bromovirus replication proteins support template-selective RNA replication in Saccharomyces cerevisiae
Abstract
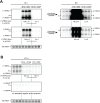
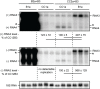
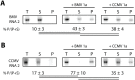
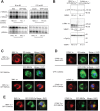
Positive-strand RNA viruses generally assemble RNA replication complexes on rearranged host membranes. Alphaviruses, other members of the alpha-like virus superfamily, and many other positive-strand RNA viruses invaginate host membrane into vesicular RNA replication compartments, known as spherules, whose interior is connected to the cytoplasm. Brome mosaic virus (BMV) and its close relative, cowpea chlorotic mottle virus (CCMV), form spherules along the endoplasmic reticulum. BMV spherule formation and RNA replication can be fully reconstituted in S. cerevisiae, enabling many studies identifying host factors and viral interactions essential for these processes. To better define and understand the conserved, core pathways of bromovirus RNA replication, we tested the ability of CCMV to similarly support spherule formation and RNA replication in yeast. Paralleling BMV, we found that CCMV RNA replication protein 1a was the only viral factor necessary to induce spherule membrane rearrangements and to recruit the viral 2a polymerase (2apol) to the endoplasmic reticulum. CCMV 1a and 2apol also replicated CCMV and BMV genomic RNA2, demonstrating core functionality of CCMV 1a and 2apol in yeast. However, while BMV and CCMV 1a/2apol strongly replicate each others' genomic RNA3 in plants, neither supported detectable CCMV RNA3 replication in yeast. Moreover, in contrast to plant cells, in yeast CCMV 1a/2apol supported only limited replication of BMV RNA3 (<5% of that by BMV 1a/2apol). In keeping with this, we found that in yeast CCMV 1a was significantly impaired in recruiting BMV or CCMV RNA3 to the replication complex. Overall, we show that many 1a and 2apol functions essential for replication complex assembly, and their ability to be reconstituted in yeast, are conserved between BMV and CCMV. However, restrictions of CCMV RNA replication in yeast reveal previously unknown 1a-linked, RNA-selective host contributions to the essential early process of recruiting viral RNA templates to the replication complex.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates.J Virol. 2005 Nov;79(21):13747-58. doi: 10.1128/JVI.79.21.13747-13758.2005. J Virol. 2005. PMID: 16227294 Free PMC article.
-
Use of bromovirus RNA2 hybrids to map cis- and trans-acting functions in a conserved RNA replication gene.J Virol. 1990 Jan;64(1):69-77. doi: 10.1128/JVI.64.1.69-77.1990. J Virol. 1990. PMID: 2293671 Free PMC article.
-
Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum.J Virol. 1999 Dec;73(12):10303-9. doi: 10.1128/JVI.73.12.10303-10309.1999. J Virol. 1999. PMID: 10559348 Free PMC article.
-
Bromovirus-induced remodeling of host membranes during viral RNA replication.Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review.
-
Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication.Annu Rev Phytopathol. 2003;41:77-98. doi: 10.1146/annurev.phyto.41.052002.095717. Epub 2003 Mar 10. Annu Rev Phytopathol. 2003. PMID: 12651962 Review.
Cited by
-
Characterization of Variant RNAs Encapsidated during Bromovirus Infection by High-Throughput Sequencing.Pathogens. 2024 Jan 22;13(1):96. doi: 10.3390/pathogens13010096. Pathogens. 2024. PMID: 38276169 Free PMC article.
-
A conserved viral amphipathic helix governs the replication site-specific membrane association.PLoS Pathog. 2022 Sep 1;18(9):e1010752. doi: 10.1371/journal.ppat.1010752. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36048900 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

