Dissociation of membrane-chromatin contacts is required for proper chromosome segregation in mitosis
- PMID: 30586323
- PMCID: PMC6594442
- DOI: 10.1091/mbc.E18-10-0609
Dissociation of membrane-chromatin contacts is required for proper chromosome segregation in mitosis
Abstract
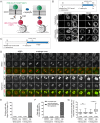
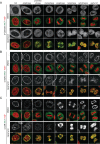
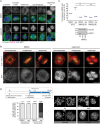
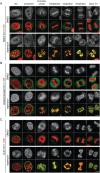
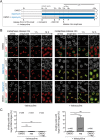
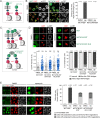
The nuclear envelope (NE) aids in organizing the interphase genome by tethering chromatin to the nuclear periphery. During mitotic entry, NE-chromatin contacts are broken. Here, we report on the consequences of impaired NE removal from chromatin for cell division of human cells. Using a membrane-chromatin tether that cannot be dissociated when cells enter mitosis, we show that a failure in breaking membrane-chromatin interactions impairs mitotic chromatin organization, chromosome segregation and cytokinesis, and induces an aberrant NE morphology in postmitotic cells. In contrast, chromosome segregation and cell division proceed successfully when membrane attachment to chromatin is induced during metaphase, after chromosomes have been singularized and aligned at the metaphase plate. These results indicate that the separation of membranes and chromatin is critical during prometaphase to allow for proper chromosome compaction and segregation. We propose that one cause of these defects is the multivalency of membrane-chromatin interactions.
Figures

Similar articles
-
Torsin ATPases influence chromatin interaction of the Torsin regulator LAP1.Elife. 2020 Dec 15;9:e63614. doi: 10.7554/eLife.63614. Elife. 2020. PMID: 33320087 Free PMC article.
-
REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture.Dev Cell. 2013 Aug 12;26(3):315-23. doi: 10.1016/j.devcel.2013.06.016. Epub 2013 Aug 1. Dev Cell. 2013. PMID: 23911198 Free PMC article.
-
Telomere-nuclear envelope dissociation promoted by Rap1 phosphorylation ensures faithful chromosome segregation.Curr Biol. 2012 Oct 23;22(20):1932-7. doi: 10.1016/j.cub.2012.08.019. Epub 2012 Sep 6. Curr Biol. 2012. PMID: 22959349
-
The coordination of nuclear envelope assembly and chromosome segregation in metazoans.Nucleus. 2020 Dec;11(1):35-52. doi: 10.1080/19491034.2020.1742064. Nucleus. 2020. PMID: 32208955 Free PMC article. Review.
-
Reorganization of the nuclear envelope during open mitosis.Curr Opin Cell Biol. 2008 Dec;20(6):669-77. doi: 10.1016/j.ceb.2008.09.010. Epub 2008 Nov 2. Curr Opin Cell Biol. 2008. PMID: 18938243 Free PMC article. Review.
Cited by
-
Nuclear envelope remodelling during mitosis.Curr Opin Cell Biol. 2021 Jun;70:67-74. doi: 10.1016/j.ceb.2020.12.004. Epub 2021 Jan 6. Curr Opin Cell Biol. 2021. PMID: 33421755 Free PMC article. Review.
-
Endomembranes promote chromosome missegregation by ensheathing misaligned chromosomes.J Cell Biol. 2022 Jun 6;221(6):e202203021. doi: 10.1083/jcb.202203021. Epub 2022 Apr 29. J Cell Biol. 2022. PMID: 35486148 Free PMC article.
-
Cell cycle regulation of ER membrane biogenesis protects against chromosome missegregation.Dev Cell. 2021 Dec 20;56(24):3364-3379.e10. doi: 10.1016/j.devcel.2021.11.009. Epub 2021 Nov 30. Dev Cell. 2021. PMID: 34852214 Free PMC article.
-
Torsin ATPases influence chromatin interaction of the Torsin regulator LAP1.Elife. 2020 Dec 15;9:e63614. doi: 10.7554/eLife.63614. Elife. 2020. PMID: 33320087 Free PMC article.
-
Interplay of the nuclear envelope with chromatin in physiology and pathology.Nucleus. 2020 Dec;11(1):205-218. doi: 10.1080/19491034.2020.1806661. Nucleus. 2020. PMID: 32835589 Free PMC article. Review.
References
-
- Baxter J, Aragon L. (2012). A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet , 110–117. - PubMed
-
- Baxter J, Sen N, Martinez VL, De Carandini ME, Schvartzman JB, Diffley JF, Aragon L. (2011). Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science , 1328–1332. - PubMed
-
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. (2002). Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell , 83–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

