Norovirus antivirals: Where are we now?
- PMID: 30584800
- PMCID: PMC7168425
- DOI: 10.1002/med.21545
Norovirus antivirals: Where are we now?
Abstract
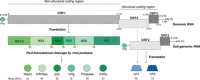
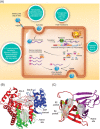
Human noroviruses inflict a significant health burden on society and are responsible for approximately 699 million infections and over 200 000 estimated deaths worldwide each year. Yet despite significant research efforts, approved vaccines or antivirals to combat this pathogen are still lacking. Safe and effective antivirals are not available, particularly for chronically infected immunocompromised individuals, and for prophylactic applications to protect high-risk and vulnerable populations in outbreak settings. Since the discovery of human norovirus in 1972, the lack of a cell culture system has hindered biological research and antiviral studies for many years. Recent breakthroughs in culturing human norovirus have been encouraging, however, further development and optimization of these novel methodologies are required to facilitate more robust replication levels, that will enable reliable serological and replication studies, as well as advances in antiviral development. In the last few years, considerable progress has been made toward the development of norovirus antivirals, inviting an updated review. This review focuses on potential therapeutics that have been reported since 2010, which were examined across at least two model systems used for studying human norovirus or its enzymes. In addition, we have placed emphasis on antiviral compounds with a defined chemical structure. We include a comprehensive outline of direct-acting antivirals and offer a discussion of host-modulating compounds, a rapidly expanding and promising area of antiviral research.
Keywords: antivirals; direct-acting antivirals; host-targeting drugs; norovirus; therapy.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Development of a broad-spectrum therapeutic Fc-nanobody for human noroviruses.J Virol. 2024 Jul 23;98(7):e0070724. doi: 10.1128/jvi.00707-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953655
-
Advances Toward a Norovirus Antiviral: From Classical Inhibitors to Lethal Mutagenesis.J Infect Dis. 2016 Feb 1;213 Suppl 1(Suppl 1):S27-31. doi: 10.1093/infdis/jiv280. J Infect Dis. 2016. PMID: 26744429 Free PMC article.
-
Advances in norovirus biology.Cell Host Microbe. 2014 Jun 11;15(6):668-80. doi: 10.1016/j.chom.2014.05.015. Cell Host Microbe. 2014. PMID: 24922570 Free PMC article. Review.
-
Norovirus: targets and tools in antiviral drug discovery.Biochem Pharmacol. 2014 Sep 1;91(1):1-11. doi: 10.1016/j.bcp.2014.05.021. Epub 2014 Jun 2. Biochem Pharmacol. 2014. PMID: 24893351 Free PMC article. Review.
-
Recent Advances in the Discovery of Norovirus Therapeutics.J Med Chem. 2015 Dec 24;58(24):9438-50. doi: 10.1021/acs.jmedchem.5b00762. Epub 2015 Aug 17. J Med Chem. 2015. PMID: 26258852 Free PMC article. Review.
Cited by
-
Norovirus NS1/2 protein increases glutaminolysis for efficient viral replication.PLoS Pathog. 2024 Jul 8;20(7):e1011909. doi: 10.1371/journal.ppat.1011909. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 38976719 Free PMC article.
-
Development of a broad-spectrum therapeutic Fc-nanobody for human noroviruses.J Virol. 2024 Jul 23;98(7):e0070724. doi: 10.1128/jvi.00707-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953655
-
A narrative review of norovirus epidemiology, biology, and challenges to vaccine development.NPJ Vaccines. 2024 May 29;9(1):94. doi: 10.1038/s41541-024-00884-2. NPJ Vaccines. 2024. PMID: 38811605 Free PMC article. Review.
-
Sapovirus: an emerging pathogen in kidney transplant recipients?Infection. 2024 Oct;52(5):1831-1838. doi: 10.1007/s15010-024-02242-9. Epub 2024 Apr 9. Infection. 2024. PMID: 38592660 Free PMC article.
-
The Scope and Impact of Viral Infections in Common Variable Immunodeficiency (CVID) and CVID-like Disorders: A Literature Review.J Clin Med. 2024 Mar 16;13(6):1717. doi: 10.3390/jcm13061717. J Clin Med. 2024. PMID: 38541942 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

