Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression
- PMID: 30581534
- PMCID: PMC6276484
- DOI: 10.1155/2018/6069150
Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression
Abstract
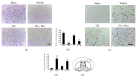
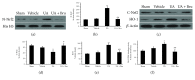
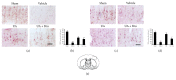
The aim of this study was to investigate whether uric acid (UA) might exert neuroprotection via activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and regulating neurotrophic factors in the cerebral cortices after transient focal cerebral ischemia/reperfusion (FCI/R) in rats. UA was intravenously injected through the tail vein (16 mg/kg) 30 min after the onset of reperfusion in rats subjected to middle cerebral artery occlusion for 2 h. Neurological deficit score was performed to analyze neurological function at 24 h after reperfusion. Terminal deoxynucleotidyl transferase-mediated dNTP nick end labeling (TUNEL) staining and hematoxylin and eosin (HE) staining were used to detect histological injury of the cerebral cortex. Malondialdehyde (MDA), the carbonyl groups, and 8-hydroxyl-2'-deoxyguanosine (8-OHdG) levels were employed to evaluate oxidative stress. Nrf2 and its downstream antioxidant protein, heme oxygenase- (HO-) 1,were detected by western blot. Nrf2 DNA-binding activity was observed using an ELISA-based measurement. Expressions of BDNF and NGF were analyzed by immunohistochemistry. Our results showed that UA treatment significantly suppressed FCI/R-induced oxidative stress, accompanied by attenuating neuronal damage, which subsequently decreased the infarct volume and neurological deficit. Further, the treatment of UA activated Nrf2 signaling pathway and upregulated BDNF and NGF expression levels. Interestingly, the aforementioned effects of UA were markedly inhibited by administration of brusatol, an inhibitor of Nrf2. Taken together, the antioxidant and neuroprotective effects afforded by UA treatment involved the modulation of Nrf2-mediated oxidative stress and regulation of BDNF and NGF expression levels. Thus, UA treatment could be of interest to prevent FCI/R injury.
Figures


Similar articles
-
Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3.Brain Res. 2015 Apr 24;1605:39-48. doi: 10.1016/j.brainres.2015.02.015. Epub 2015 Feb 16. Brain Res. 2015. PMID: 25698615
-
5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia.Cell Stress Chaperones. 2017 Jan;22(1):55-65. doi: 10.1007/s12192-016-0742-0. Epub 2016 Nov 3. Cell Stress Chaperones. 2017. PMID: 27812888 Free PMC article.
-
Protocatechualdehyde Protects Against Cerebral Ischemia-Reperfusion-Induced Oxidative Injury Via Protein Kinase Cε/Nrf2/HO-1 Pathway.Mol Neurobiol. 2017 Mar;54(2):833-845. doi: 10.1007/s12035-016-9690-z. Epub 2016 Jan 16. Mol Neurobiol. 2017. PMID: 26780453
-
[Research progress of nuclear factor E 2-related factor 2 signaling pathway in neuroprotective mechanism of cerebral ischemia/reperfusion injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Mar;34(3):325-328. doi: 10.3760/cma.j.cn121430-20211018-01506. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 35574756 Review. Chinese.
-
Mitochondrial mechanisms in Cerebral Ischemia-Reperfusion Injury: Unravelling the intricacies.Mitochondrion. 2024 Jul;77:101883. doi: 10.1016/j.mito.2024.101883. Epub 2024 Apr 15. Mitochondrion. 2024. PMID: 38631511 Review.
Cited by
-
Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages.Molecules. 2022 Feb 3;27(3):1049. doi: 10.3390/molecules27031049. Molecules. 2022. PMID: 35164314 Free PMC article.
-
Association Between Serum Uric Acid and Intracranial Arterial Stenosis in a Korean Population: A Secondary Analysis Based on a Cross-Sectional Study.Front Neurol. 2022 Mar 11;13:791456. doi: 10.3389/fneur.2022.791456. eCollection 2022. Front Neurol. 2022. PMID: 35359641 Free PMC article.
-
The Prognostic Significance of Uric Acid/Albumin Ratio in Patients with Aortic Stenosis Following Transcatheter Aortic Valve Implantation for Major Adverse Cardiac and Cerebral Events.Medicina (Kaunas). 2023 Mar 30;59(4):686. doi: 10.3390/medicina59040686. Medicina (Kaunas). 2023. PMID: 37109644 Free PMC article.
-
Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO-1 signaling pathway in mice.Fundam Clin Pharmacol. 2022 Oct;36(5):790-800. doi: 10.1111/fcp.12782. Epub 2022 May 4. Fundam Clin Pharmacol. 2022. PMID: 35470467 Free PMC article.
-
Evaluation of Oxidative Status in Elderly Patients with Multiple Cerebral Infarctions and Multiple Chronic Total Coronary Occlusions.Dis Markers. 2022 Jun 28;2022:2083990. doi: 10.1155/2022/2083990. eCollection 2022. Dis Markers. 2022. PMID: 35801004 Free PMC article.
References
-
- Janyou A., Wicha P., Jittiwat J., Suksamrarn A., Tocharus C., Tocharus J. Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Scientific Reports. 2017;7(1, article 10556) doi: 10.1038/s41598-017-11181-5. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

