Emerging Insights into the Function of Kinesin-8 Proteins in Microtubule Length Regulation
- PMID: 30577528
- PMCID: PMC6359247
- DOI: 10.3390/biom9010001
Emerging Insights into the Function of Kinesin-8 Proteins in Microtubule Length Regulation
Abstract
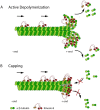
Proper regulation of microtubules (MTs) is critical for the execution of diverse cellular processes, including mitotic spindle assembly and chromosome segregation. There are a multitude of cellular factors that regulate the dynamicity of MTs and play critical roles in mitosis. Members of the Kinesin-8 family of motor proteins act as MT-destabilizing factors to control MT length in a spatially and temporally regulated manner. In this review, we focus on recent advances in our understanding of the structure and function of the Kinesin-8 motor domain, and the emerging contributions of the C-terminal tail of Kinesin-8 proteins to regulate motor activity and localization.
Keywords: microtubule dynamics; mitosis; molecular motor protein; spindle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Spatial regulation of astral microtubule dynamics by Kif18B in PtK cells.Mol Biol Cell. 2016 Oct 15;27(20):3021-3030. doi: 10.1091/mbc.E16-04-0254. Epub 2016 Aug 24. Mol Biol Cell. 2016. PMID: 27559136 Free PMC article.
-
Discrete regions of the kinesin-8 Kip3 tail differentially mediate astral microtubule stability and spindle disassembly.Mol Biol Cell. 2018 Aug 1;29(15):1866-1877. doi: 10.1091/mbc.E18-03-0199. Epub 2018 Jun 6. Mol Biol Cell. 2018. PMID: 29874146 Free PMC article.
-
Kinesin-8 motors: regulation of microtubule dynamics and chromosome movements.Chromosoma. 2020 Jun;129(2):99-110. doi: 10.1007/s00412-020-00736-7. Epub 2020 May 17. Chromosoma. 2020. PMID: 32417983 Review.
-
Kinesin-8 effects on mitotic microtubule dynamics contribute to spindle function in fission yeast.Mol Biol Cell. 2016 Nov 7;27(22):3490-3514. doi: 10.1091/mbc.E15-07-0505. Epub 2016 May 4. Mol Biol Cell. 2016. PMID: 27146110 Free PMC article.
-
Kinesin-13s in mitosis: Key players in the spatial and temporal organization of spindle microtubules.Semin Cell Dev Biol. 2010 May;21(3):276-82. doi: 10.1016/j.semcdb.2010.01.016. Epub 2010 Jan 28. Semin Cell Dev Biol. 2010. PMID: 20109574 Free PMC article. Review.
Cited by
-
Plasmodium kinesin-8X associates with mitotic spindles and is essential for oocyst development during parasite proliferation and transmission.PLoS Pathog. 2019 Oct 10;15(10):e1008048. doi: 10.1371/journal.ppat.1008048. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31600347 Free PMC article.
-
13 Plus 1: A 30-Year Perspective on Microtubule-Based Motility in Dictyostelium.Cells. 2020 Feb 25;9(3):528. doi: 10.3390/cells9030528. Cells. 2020. PMID: 32106406 Free PMC article. Review.
-
Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform.Biomolecules. 2020 Apr 7;10(4):563. doi: 10.3390/biom10040563. Biomolecules. 2020. PMID: 32272590 Free PMC article.
-
Microtubule Organization in Striated Muscle Cells.Cells. 2020 Jun 3;9(6):1395. doi: 10.3390/cells9061395. Cells. 2020. PMID: 32503326 Free PMC article. Review.
-
New insights into the mechanochemical coupling mechanism of kinesin-microtubule complexes from their high-resolution structures.Biochem Soc Trans. 2023 Aug 31;51(4):1505-1520. doi: 10.1042/BST20221238. Biochem Soc Trans. 2023. PMID: 37560910 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

