INASL Guidelines on Management of Hepatitis B Virus Infection in Patients receiving Chemotherapy, Biologicals, Immunosupressants, or Corticosteroids
- PMID: 30568345
- PMCID: PMC6286881
- DOI: 10.1016/j.jceh.2018.06.010
INASL Guidelines on Management of Hepatitis B Virus Infection in Patients receiving Chemotherapy, Biologicals, Immunosupressants, or Corticosteroids
Abstract
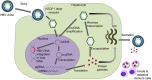
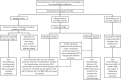
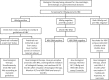
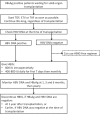
Hepatitis B Virus (HBV) reactivation in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids is emerging to be an important cause of morbidity and mortality in patients with current or prior exposure to HBV infection. These patients suffer a dual onslaught of illness: one from the primary disease for which they are receiving the culprit drug that led to HBV reactivation, and the other from HBV reactivation itself. The HBV reactivation not only leads to a compromised liver function, which may culminate into hepatic failure; it also adversely impacts the treatment outcome of the primary illness. Hence, identification of patients at risk of reactivation before starting these drugs, and starting treatment aimed at prevention of HBV reactivation is the best strategy of managing these patients. There are no Indian guidelines on management of HBV infection in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids for the treatment of rheumatologic conditions, malignancies, inflammatory bowel disease, dermatologic conditions, or solid-organ or bone marrow transplantation. The Indian National Association for Study of the Liver (INASL) had set up a taskforce on HBV in 2016, with a mandate to develop consensus guidelines for management of various aspects of HBV infection, relevant to India. In 2017 the taskforce had published the first INASL guidelines on management of HBV infection in India. In the present guidelines, which are in continuation with the previous guidelines, the issues on management of HBV infection in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids are addressed.
Keywords: ACLF, Acute-on-Chronic Liver Failure; AFP, Alphafetoprotein; ALT, Alanine Aminotransferase; Anti-HBc, Antibodies to Hepatitis B Core Antigen; Anti-HBs, Antibodies to Hepatitis B Surface Antigen; CHB, Chronic Hepatitis B; CHOP, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone; CKD, Chronic Kidney Disease; DILI, Drug-Induced Liver Injury; DNA, Deoxyribonucleic Acid; ETV, Entecavir; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; HAV, Hepatitis A Virus; HBIG, Hepatitis B Immune Globulin; HBV DNA, Hepatitis B Virus Deoxyribonucleic Acid; HBV, Hepatitis B Virus; HBcAg, Hepatitis B Core Antigen; HBeAg, Hepatitis B Envelope Antigen; HBsAg, Hepatitis B Surface Antigen; HDV, Hepatitis D Virus; HEV, Hepatitis E Virus; HLA, Human Leukocyte Antigen Class I; INASL, Indian National Association for Study of the Liver; LAM, Lamivudine; NAs, Nucleos(t)ide Analogs; NHL, Non-Hodgkin’s Lymphoma; NK, Natural Killer; PegIFN-α, Pegylated Interferon Alpha; RA, Rheumatoid Arthritis; SLE, Systemic Lupus Erythematosus; TAF, Tenofovir Alafenamide; TDF, Tenofovir Disoproxil Fumarate; TLC, Total Leucocyte Count; ULN, Upper Limit of Normal; cancer; cccDNA, Covalently Closed Circular Deoxyribonucleic Acid; chemotherapy; hepatitis B; immunosupressants; liver failure; rcDNA, Relaxed-Circular Deoxyribonucleic Acid.
Figures

Similar articles
-
INASL Position Statements on Prevention, Diagnosis and Management of Hepatitis B Virus Infection in India: The Andaman Statements.J Clin Exp Hepatol. 2018 Mar;8(1):58-80. doi: 10.1016/j.jceh.2017.12.001. Epub 2017 Dec 16. J Clin Exp Hepatol. 2018. PMID: 29743798 Free PMC article. Review.
-
Indian National Association for the Study of the Liver-Federation of Obstetric and Gynaecological Societies of India Position Statement on Management of Liver Diseases in Pregnancy.J Clin Exp Hepatol. 2019 May-Jun;9(3):383-406. doi: 10.1016/j.jceh.2019.02.007. Epub 2019 Mar 6. J Clin Exp Hepatol. 2019. PMID: 31360030 Free PMC article. Review.
-
Clinical Profile and Efficacy of Antivirals in Hepatitis B Virus Reactivation, in Patients With Cancer Receiving Chemotherapy.J Clin Exp Hepatol. 2020 Nov-Dec;10(6):590-598. doi: 10.1016/j.jceh.2020.06.008. Epub 2020 Jul 8. J Clin Exp Hepatol. 2020. PMID: 33311896 Free PMC article.
-
Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective.J Clin Virol. 2013 Sep;58(1):67-73. doi: 10.1016/j.jcv.2013.06.035. Epub 2013 Jul 20. J Clin Virol. 2013. PMID: 23880162
-
Rebound of HBV DNA after cessation of nucleos/tide analogues in chronic hepatitis B patients with undetectable covalently closed.JHEP Rep. 2020 Mar 29;2(3):100112. doi: 10.1016/j.jhepr.2020.100112. eCollection 2020 Jun. JHEP Rep. 2020. PMID: 32462119 Free PMC article.
Cited by
-
DAPS score - a novel score for prediction of significant fibrosis in incidentally detected asymptomatic hepatitis B subjects.Clin Exp Hepatol. 2023 Mar;9(1):9-13. doi: 10.5114/ceh.2023.124518. Epub 2023 Feb 14. Clin Exp Hepatol. 2023. PMID: 37064839 Free PMC article.
-
Should We Treat Immune Tolerant Chronic Hepatitis B? Lessons from Asia.J Clin Exp Hepatol. 2022 Jan-Feb;12(1):144-154. doi: 10.1016/j.jceh.2021.08.023. Epub 2021 Aug 27. J Clin Exp Hepatol. 2022. PMID: 35068795 Free PMC article. Review.
-
A comprehensive review of vaccination in patients with inflammatory bowel diseases: An Indian perspective.Indian J Gastroenterol. 2020 Aug;39(4):321-330. doi: 10.1007/s12664-020-01069-0. Epub 2020 Aug 26. Indian J Gastroenterol. 2020. PMID: 32844299 Free PMC article. Review.
-
Managing HBV and HCV Infection Pre- and Post-liver Transplant.J Clin Exp Hepatol. 2024 Mar-Apr;14(2):101287. doi: 10.1016/j.jceh.2023.09.008. Epub 2023 Sep 23. J Clin Exp Hepatol. 2024. PMID: 38076445 Review.
-
HBV Reactivation in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Narrative Review.Viruses. 2019 Nov 10;11(11):1049. doi: 10.3390/v11111049. Viruses. 2019. PMID: 31717647 Free PMC article. Review.
References
-
- Arora A. INASL position statements on prevention, diagnosis and management of hepatitis B virus infection in India: the Andaman Statements. J Clin Exp Hepatol. 2018 https://doi.org/10.1016/j.jceh.2017.12.001. - PMC - PubMed
-
- Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet Lond Engl. 2015;386(10003):1546–1555. https://doi.org/10.1016/S0140-6736(15)61412-X. - PubMed
-
- Ott J.J., Horn J., Krause G., Mikolajczyk R.T. Time trends of chronic HBV infection over prior decades – global analysis. J Hepatol. 2017;66(1):48–54. https://doi.org/10.1016/j.jhep.2016.08.013. - PubMed
-
- Stanaway J.D., Flaxman A.D., Naghavi M. The Global Burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet Lond Engl. 2016;388(10049):1081–1088. https://doi.org/10.1016/S0140-6736(16)30579-7. - PMC - PubMed
-
- WHO | Hepatitis. WHO. http://www.who.int/immunization/topics/hepatitis/en/. Accessed January 24, 2018.
LinkOut - more resources
Full Text Sources
Research Materials

